Abstract
Introduction
Spontaneous reporting systems (SRSs) are pivotal for signal detection, especially for rare events with a high drug-attributable component, such as torsade de pointes (TdP). Use of different national SRSs is rarely attempted because of inherent difficulties, but should be considered on the assumption that rare events are diluted in international databases.
Objective
The aim was to describe TdP-related events associated with antipsychotics, H1-antihistamines and anti-infectives in three national SRSs (in Italy, Germany and France) and highlight potential signals of torsadogenicity through a combined literature evaluation.
Methods
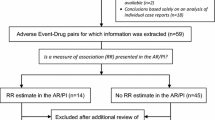
A common search strategy was applied to extract TdP-related events: (1) TdP, (2) QT interval abnormalities, (3) ventricular fibrillation/tachycardia, and (4) sudden cardiac death. Signals of disproportionate reporting (SDRs) were calculated for TdP + QT interval abnormalities and defined by a lower limit of the 95 % confidence interval of the reporting odds ratio (ROR) >1. Among SDRs with at least three cases without concomitant pro-arrhythmic drugs, we defined potential new signal of torsadogenicity as drugs with no published evidence from (a) the crediblemeds® website (http://www.crediblemeds.com, as of November 1st, 2014); (b) studies on the FDA Adverse Event Reporting System (FAERS); and (c) safety trials or pharmaco-epidemiological studies (as of December 16th, 2014).
Results
Overall, 3505 cases were retrieved (1372, 1468, and 801 for France, Germany and Italy, respectively). Antipsychotics were mainly recorded in Germany (792 cases), whereas antibiotics peaked at 515 and 491 (France and Italy, respectively). Forty-one drugs met criteria for SDRs in at least one single source, of which 31 were detected only from one single SRS: 18, ten and three (French, German and Italian SRS, respectively). By contrast, only five SDRs were detected in all national data sources (amisulpride, aripiprazole, haloperidol, olanzapine, risperidone). Overall, five potential new signals of torsadogenicity were identified: flupentixol, ganciclovir, levocetirizine, oxatomide and tiapride.
Conclusions
We found differences across and within national SRSs in the reporting of drug-induced TdP, which finally resulted in five potential new signals of torsadogenicity. These findings warrant targeted pharmacovigilance studies to formally assess the existence of actual drug–event associations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Diversity across and within national spontaneous reporting systems is likely to be multifactorial but informative of the local reporting pattern of drug-induced arrhythmia. |
Five potential signals, undetected by recent studies in the FDA Adverse Event Reporting System, warrant validation through additional post-marketing sources, namely analytical pharmaco-epidemiological approaches. |
In the era of large international spontaneous reporting systems, we provide preliminary evidence on the role of national databases in detecting rare adverse drug reactions, at least for drugs with well-established use. |
1 Introduction
Torsade de pointes (TdP) is a rare but potentially fatal arrhythmia characterized by a marked drug-attributable component; its suboptimal prediction and detection in pre-marketing phases of drug development caused a number of regulatory interventions worldwide, including drug withdrawals, restrictions and warnings, thus making spontaneous reporting systems (SRSs) pivotal in signal detection. The post-marketing epidemic caused by non-cardiac drugs with TdP liability has triggered a global response from drug regulators, drug developers and academia, which resulted in the stabilization of the reporting rate of TdP [1].
Within the ARITMO project (http://www.aritmo-project.org), both international and national SRSs have been exploited to comprehensively collect and analyze the torsadogenic liability of antipsychotics, H1-antihistamines and anti-infectives for systemic use, with the ultimate goal of capturing potential signals of torsadogenicity requiring population-based studies and possible regulatory consideration.
National databases have been used very rarely to analyze the risk of drug-induced TdP in the post-marketing setting [2]; this may depend on the fact that a single national SRS is perceived to be insufficient to detect rare events, especially as compared with the large catchment area of international databases, such as the FDA Adverse Event Reporting System (FAERS). Nonetheless, national databases have the advantage of providing the actual local picture of the risk (which depends on the real drug use) and offer access to the patient’s medical history (the so-called “narratives”), thus potentially enhancing the performance of signal detection. In particular, multiple database analysis can be used to compare results across databases, while maintaining the diversity of reporting pattern within each single source, and avoid the theoretical dilution phenomenon that may occur when analyzing international SRSs [3].
On these grounds, we analyzed three European national SRSs (i.e., French, German and Italian databases), with the following aims: (1) to describe the distribution of TdP-related events associated with antipsychotics, H1-antihistamines and anti-infectives among the different databases; and (2) to identify novel signals of torsadogenicity, by comparing published literature data, especially from international SRSs, namely FAERS.
2 Methods
2.1 Data Sources: Accessibility and Technical Issues
A summary of national SRSs is provided below, with a description of the key technical issues faced to harmonize the process of data extraction. In all SRSs, drugs are codified through the Anatomical Therapeutic Chemical (ATC) Classification System codes, whereas adverse reports use Medical Dictionary for Regulatory Activities (MedDRA®) terms. Within each database, the in-house de-duplication process was applied.
-
1.
The French SRS (2000–2010), named the Base Nationale de Pharmacovigilance (BNPV), is a computerized information database for research purposes, and includes medically validated reports from 31 regional pharmacovigilance centers; no data are collected from the manufacturer. Data submitted to the BNPV are stored in 14 different tables that are linked to each other using the “report identification number”. The French Imputability Method is adopted for causality assessment [4]. Reports related to vaccines are collected only in the French database and, therefore, were identified and excluded if vaccine(s) was the only reported agent. Likewise, reports on illicit drugs, heavy metals intoxications, homeopathic treatments, herbal medicines, medical devices, cosmetics, and accidental ingestion of substances that are not strictly considered as medication (e.g., mouthwash) were excluded [5].
-
2.
The German SRS (2005–2010) collects all adverse events associated with all licensed medicinal products on the market in Germany; it is operated by the Federal Institute for Drugs and Medical Devices (German name, BfArM), which usually accepts reports from healthcare professionals only. However, reports submitted by pharmaceutical industries have been recorded since 2008. The database is accessible for research purposes, provided that a formal application with a defined subset of variables is presented to the Agency. Causality of drug-induced adverse events is only suspected and not validated. Data coverage starts in 1978, although full electronic records are only available from 2005 onwards. Free-text search strategy cannot be performed. Manual codification of drugs was specifically implemented to systematically codify active substances into V level ATC codes.
-
3.
The Italian SRS (1969–2010) is based on the Rete Nazionale di Farmacovigilanza (RNF), a network involving the Italian Medicines Agency (AIFA), Italian regions, local health units, hospitals, Institute of Research and Care and drug industries. Regional pharmacovigilance centers are responsible for causality assessment and quality control of submitted data. Each case report is available to each operator within the RNF in line with the authorization for his/her type of account. For every data entry or data update in the national database, the relevant Market Authorization Holder is notified to avoid duplicate sending to Eudravigilance. The drug manufacturer has the obligation to send reports from the literature of serious adverse drug reaction (ADR) occurring in Italy to the RNF, attaching the relevant article. Reports coming from the literature have not been considered in the analyses. Codification of adverse reactions has been performed through MedDRA® since 2005, whereas for the 1969–2004 period, the World Health Organization Adverse Reaction Terminology (WHO–ART) terminology was used; a bridge is required to search the database with both terminologies.
2.2 Case and Exposure Definition
A protocol was developed to extract all potential torsadogenic events suspected to be attributed to antipsychotics (ATC code N05A), antihistamines (R06) and anti-infectives, namely antibiotics (J01), antimycotics (J02), antimycobacterials (J04), antivirals (J05) and antiprotozoals (P01). As previously detailed, four mutually exclusive groups of events of interest were defined to fully capture the heterogeneous clinical nature of drug-induced TdP, in decreasing order of drug-attributable risk. Subcategories were created regarding the severity of the outcome, that is, whether the event caused death or life-threatening events. These groups were (1) TdP, (2A) symptomatic QT abnormalities, (2B) asymptomatic QT interval abnormalities, (3A) ventricular/cardiac fibrillation, (3B) ventricular tachycardia/arrhythmia (fatal/serious), (3C) ventricular tachycardia/arrhythmia (non-fatal/serious), (4A) sudden cardiac death/cardiac arrest, (4B) syncope (fatal/serious). Details on outcome definition and relevant codification have been fully provided elsewhere [6]. In the Italian and French databases, a free-text search strategy was also performed by analyzing narratives through automatic search, based on a list of “string” and/or items agreed among authors (see the Electronic Supplementary Material, Table S1). These potential additional cases were finally validated for inclusion by a clinical pharmacologist and/or pharmacologist with expertise in pharmacovigilance (i.e., F.S. and S.A. for the French database; U.M. for the Italian database).
2.3 Signal Detection Approach
First, we performed a disproportionality analysis in terms of drug–case pairs and considering drugs recorded as “suspect”. We calculated the reporting odds ratio (ROR) with the relevant 95 % confidence interval (95 % CI); signal of disproportionate reporting (SDR) was defined when at least three cases of interest were recorded and a statistically significant ROR emerged (i.e., lower limit of the 95 % CI >1) [7]. Considering the high degree of drug-attributable risk of TdP and its recognized relationship, albeit not straightforward, with QT prolongation, we focused disproportionality analysis on group 1 + 2 (i.e., TdP + QT interval abnormalities).
Second, we selected SDRs (group 1 + 2) with also no concomitant use of cardiovascular drugs, including class I/III antiarrhythmics, or agents listed by the crediblemeds® website (http://www.crediblemeds.com, as of June 2011, when the analysis was performed), which may act both as confounders of the drug–event association and may also increase the likelihood of TdP occurrence. An SDR was defined as “substantiated” if at least three cases without these concomitant agents were reported, a “qualitative” evaluation that takes into account the multi-hit hypothesis in the genesis of TdP [8, 9].
Third, a combined literature evaluation was carried out to assess the novelty of the association (i.e., unexpectedness). A potential signal was finally defined if a drug fulfilled all the following criteria:
-
No mention by the crediblemeds ® website (http://www.crediblemeds.com, as of November 1st, 2014). All the three lists were checked.
-
No published pharmacovigilance evidence from international SRSs The publicly available FAERS was identified as the comparator, considering that all pharmacological classes of interest have been covered in the recent literature using the same search strategy and a comparable time window [6, 8, 10–12]. Absence of pharmacovigilance evidence was defined by negative SDRs (i.e., the ROR did not exceed the threshold for statistical significance or less than three cases were reported).
-
No consolidated clinical evidence Published data in humans were used, in particular those derived from analytical pharmaco-epidemiological studies, and thorough QT studies submitted for marketing approval. Case reports were not considered (literature search as of December 16th, 2014). Absence of clinical evidence was defined by negative thorough QT study or clinical study documenting only mild-to-moderate QT prolongation without occurrence of arrhythmia.
3 Results
3.1 Descriptive Analysis
Overall, 3505 cases for events of interest and ARITMO drug classes were retrieved from the three national SRSs, with Germany ranked first (n = 1468). Large inter- and intra-database differences were found (Table 1). The distribution of the events in France has remarkable peculiarities: the largest volume of reports is related to group 3A (i.e., ventricular fibrillation), whereas only a few cases (if any at all) were recorded for groups 3B and 3C. Conversely, group 3C records the largest number of reports extracted in both the German and Italian SRSs. The contribution of narratives is appreciable, especially for group 1 + 2, both in Italy (23 %) and France (15 %).
Overall, France ranked first (n = 143) in terms of drugs with at least one case of interest across outcomes, followed by Italy (n = 130) and Germany (n = 110). Antibacterials (J01) were more frequently recorded in groups 3 + 4, and represented 61 % (491 out of 801) in the Italian SRS and 38 % (515 out of 1372) in the German SRS. Conversely, cases related to antipsychotics mostly emerged in the German SRS (where they ranked first, both for groups 1 + 2 and 3 + 4), and were recorded in 54 % of total cases (792 out of 1468). Antihistamines are also widely reported, especially in German and French SRSs (Table 2). The complete list of drugs with all data separated for each single outcome is provided as electronic supplementary material (see supplementary appendices S2, S3 and S4 for France, Germany and Italy, respectively).
3.2 Signal Detection
Forty-four drugs received at least three cases in at least one national SRS (Table 3), 41 of which met criteria for SDRs in at least one single source. Among these, 31 SDRs were detected only from one single national database: 18, ten and three from the French, German and Italian SRSs, respectively. Only five SDRs were detected in all national data sources (amisulpride, aripiprazole, haloperidol, olanzapine, risperidone).
Nine SDRs detected from national SRSs were characterized by at least three cases without concomitant pro-arrhythmic drugs (signal substantiation): quinine, tiapride (France), flupentixol, ganciclovir, levocetirizine, melperone, pipamperone (Germany), oxatomide (Italy), and roxithromycin (France and Germany). A detailed signal assessment is provided in Table 4. The combined literature evaluation showed that three agents (i.e., pipamperone, quinine and roxithromycin) were already listed by crediblemeds® website, whereas melperone was the only drug with published clinical evidence (see Sect. 4). All agents were not captured by disproportionality analyses conducted in FAERS by previous recent studies. Therefore, based on pre-specified criteria, five possible new signals of torsadogenicity were identified: flupentixol, ganciclovir, levocetirizine, oxatomide and tiapride.
4 Discussion
Our study aimed at testing heterogeneity among national SRSs and verifying whether this feature actually translates into improvement of signal detection performance. To our knowledge, this is the first study comparing three national SRSs for signal detection of drug-induced TdP. Diversity across and within national SRSs emerged in terms of distribution of TdP-related events and associated drugs; surprisingly, nine SDRs captured by national SRSs were not detected by the most recent studies on spontaneous reporting carried out in FAERS.
Our initial hypothesis, indeed, was that spontaneous reports in national SRSs (and submitted to FAERS) may be diluted in FAERS, and cannot be fully appreciated in international SRSs, thus, potentially compromising signal detection performance. This hypothesized dilution phenomenon may be theoretically ascribable to two major issues: (1) only serious reports from European countries are submitted to FAERS; (2) the background for comparison may be influenced by a number of biases, including masking effect, which was documented in FAERS and is hard to manage by simple unmasking protocols because of the large size and diversity of the database, characterized by complex interdependencies between drugs and events [13]. Actually, it is interesting to observe that the number of cases obtained from a single national SRS largely exceeded those retrieved from studies performed on the entire FAERS (with the exception of levocetirizine). Therefore, the most plausible interpretation is that only a minority of European reports recorded in national databases are submitted to FAERS. In other words, only partial, but unmeasured, overlap is likely to exist between national SRSs and FAERS, an unexpected phenomenon considering the seriousness of TdP and related clinical events.
Differences among national databases in case and drugs distribution (e.g., ventricular fibrillation was most frequently reported in France; antipsychotics accounted for the majority of reports in Germany, whereas anti-infectives did in Italy) are likely to be multifactorial. A driving factor is expected to be the actual pattern of drug use at the national level, which is further influenced by marketing penetration, pharmaceutical pressure on a given product(s), specific safety issues causing regulatory restrictions, number of drugs available on the market, marketing life, reimbursement issues and prevalence of the different diseases. In addition, further reasons may be the different time windows used across databases (only the Italian SRS strongly differed from the others and collects data since 1969), as well as peculiarities in drug selection, recording, mapping, detection and removal of duplicates, and methods used for causality assessment within each database. The type of reporter could also vary among SRSs. For example, in France, the majority of reports are submitted by hospital physicians and then validated by hospital practitioners specialized in pharmacovigilance. Therefore, tachycardia not leading to a hospitalization (mainly corresponding to the group 3C) is not usually reported. Moreover, the diagnosis of the cardiac disorder is performed mainly in hospital and confirmed by a specialist consultation before notification is sent to the regional center. Finally, the free-text search strategy through narratives was not feasible for the German SRS. The high rate of reports retrieved through narratives in the French SRS is due to the presence of a detailed summary of the cases (with biological exams or electrocardiogram (ECG) results), which is routinely done by the pharmacovigilance experts in French regional centers. Taken together, all these aspects make each SRS a unique entity for research and may also explain why four out of five novel signals were identified only in one database. Therefore, multiple national database analysis appears informative when studying the arrhythmogenic potential of drugs, in order to capture the entire spectrum of drug-induced torsadogenicity.
Our study should be interpreted with caution, in the light of recognized limitations affecting SRSs [14]. In particular, we acknowledge that the lack of complete patient-related risk factors and information on drug administration do not allow a full causality assessment of individual reports. In addition, although a common protocol was adopted, the aforementioned peculiarities affecting each SRS (e.g., population coverage, completeness of data, duplicate detection, drug and adverse event coding, possibility to access patient’s medical history) did not allow final data aggregation. Nonetheless, we believe that this issue represents an important strength of the research, which clearly emphasized the identity and unique capabilities of national databases when investigating the torsadogenic risk of drugs. Moreover, we cannot assess and compare the performance in signal detection among national SRSs in terms of time to first detection of a signal and the so-called false discovery rate [15]. The former was not the core of this work, and it is also believed to be a minor issue as we have analyzed drugs on the market for several years. The latter was not a pre-specified purpose of this study, it implies the identification of reference compounds/events a priori, and depends on a number of factors: the type of event under investigation [16], threshold used for signal detection [17], the adopted algorithms, which may perform differently among databases [18], the presence of co-prescriptions [19], the comparator used for disproportion calculation [20], and the nature and size of the database (e.g., national vs. international SRSs) [3].
We are aware that, theoretically, the five potential new signals may be false positives, especially considering the aforementioned methodological issues (common to all analyses on SRSs). In addition, there are three specific aspects to be mentioned. (1) The automatic multi-step data mining approach performed in FAERS, especially drug codification, de-duplication process and handling of missing data, may affect signal detection. There is the possibility that reports coming from national European SRSs were entered into FAERS without filling all key information (e.g., age, sex) and were therefore removed from published FAERS analyses [21]. (2) FAERS is not the only international SRS for comparison: Vigibase and Eudravigilance represent large international databases, but did not fulfill our criteria (i.e., published literature evidence) for signal detection when the study was planned. However, as previously demonstrated, FAERS reports on TdP were mostly submitted by US and European high-income/upper middle-income countries, thus, supporting the notion that FAERS data are highly informative of the global pattern of torsadogenic events [11]. Future studies should consider the use of other international sources, especially in the light of the current role of Eudravigilance, which recently started data collection also on medicinal products not approved through the centralized procedure. (3) Additional cases were extracted through free-text search. Nonetheless, we strongly believe that this was unlikely to result in false positives. The analysis of narratives represents an actual added value of our study because the manual validation of cases increases the sensitivity of the search without affecting the overall performance (manual codification of narratives is performed for all events recorded in the Italian database, thus, no major distortions are likely to occur). Although the contribution of narratives appears minor as compared with the standard approach (approximately 6 % in France and 10 % in Italy), the fact that few reports originated from free-text analysis strongly indicates the good quality of the overall codification process, which, both in Italy and France, involves a number of regional centers. Despite these limitations, we believe that the false-positive rate is likely to be low, as demonstrated by known associations detected by our analysis (i.e., drugs listed by crediblemeds® such as haloperidol), which can be considered as positive controls and support the accuracy of the method.
Our study carries important clinical, research and regulatory implications. From a clinical standpoint, antipsychotics are the most frequently reported agents, especially in Germany. Notably, amisulpride, aripiprazole, haloperidol, olanzapine and risperidone were the only drugs associated with SDRs in all databases. This consistency across all SRSs is indicative of a known pro-arrhythmic risk and may be also related to the considerable and increasing population exposure over years [11].
From a research standpoint, multiple database analyses are only rarely exploited for signal detection: we believe that the use of different SRSs represents an added value in the current era of drug safety research, where several consortia have been created to fully assess drug-related risks in the post-marketing setting. As compared with post-marketing safety studies on healthcare databases, where multiple databases are pooled to increase statistical power, spontaneous reporting databases should not be combined, but analyzed separately to maintain country-specific differences. Further research is now warranted to test the overall signal detection performance of national SRSs when studying rare events with newly approved drugs (especially in terms of time to first detection), for which data aggregation is theoretically advisable considering the centralized approval of the majority of current medicinal products.
From a regulatory standpoint, the remarkable proportion of torsadogenic reports recorded for antipsychotics in Germany deserves consideration by national authorities and may partially reflect their widespread prescriptions in nursing home residents with dementia, especially for first-generation agents with sedative properties [22, 23]. The SDR found for melperone is consistent with results from the recent prospective surveillance study in Berlin, which highlighted melperone as one of the drugs most frequently associated with TdP reports in Germany; this study further suggested a considerably higher incidence of TdP in Germany as compared with previous estimates (2.5–4 per million per year) [24].
Literature data of flupentixol and ganciclovir are scant and mainly based on a few case reports suggesting that underlying comorbidities and/or clinical conditions for which the drug is prescribed (encephalitis, immunocompromised patients) enhance patients’ susceptibility to TdP occurrence [25, 26].
The signal of levocetirizine emphasized that (a) the predictive value of thorough QT study is suboptimal [27] and (b) enantiomers and racemic mixtures may have different safety profiles because of possible differences in doses, metabolism and stereoselective targets [28]. It is worth noting that the signal arose from the German SRS, although no recent drug utilization data are publicly available. A previous European cross-national comparison study on aggregated data (2000–2005 period) reported an unexpected low use of reimbursed antihistamines, which was interpreted in the light of legislation on over-the-counter products [29]. The latest European drug utilization analysis found that levocetirizine was largely used in France, where the drug represented 53 % of the total defined daily doses of antihistamines [12].
As regards oxatomide, the Italian regulatory agency in 2010 required an update of the summary of product characteristics, with details for posology optimization and a contraindication in children aged <1 year, following potential administration errors of oral drops with risk of overdosage [30]. The case of oxatomide represents an example of how a signal detected by a national agency using a standard signal detection algorithm was managed at a local level. Therefore, we do not foresee the need for further regulatory actions or analytical pharmaco-epidemiological studies.
As regards tiapride, a literature review failed to identify consolidated clinical evidence; only a single case report is documented in an elderly patient with agitation and concomitant heart disease and bronchitis [31], with reassuring in vitro data: the inhibitory effect on potassium current was well above the reported therapeutic plasma concentrations achieved in humans [32].
5 Conclusions
We highlighted diversity within and across three national SRSs, which resulted in five potential signals undetected in FAERS. Considering that false-positive signals are possible, these findings warrant further investigations through (a) targeted analytical safety studies to formally assess these safety signals and (b) additional analysis of other international SRSs to assess the degree of overlap across databases, and finally confirm the actual value of national SRSs in signal detection.
References
Stockbridge N, Morganroth J, Shah RR, Garnett C. Dealing with global safety issues: was the response to QT-liability of non-cardiac drugs well coordinated? Drug Saf. 2013;36:167–82.
Aström-Lilja C, Odeberg JM, Ekman E, Hagg S. Drug-induced torsades de pointes: a review of the Swedish pharmacovigilance database. Pharmacoepidemiol Drug Saf. 2008;17:587–92.
Hammond IW, Gibbs TG, Seifert HA, Rich DS. Database size and power to detect safety signals in pharmacovigilance. Expert Opin Drug Saf. 2007;6:713–21.
Arimone Y, Bidault I, Dutertre JP, Gerardin M, Guy C, Haramburu F, Hillaire-Buys D, Meglio C, Penfornis C, Théophile H, Valnet-Rabier MB, Cercle de Réflexion sur l’Imputabilité (CRI). Updating the French method for the causality assessment of adverse drug reactions. Therapie. 2013;68:69–76.
Salvo F, Raschi E, Moretti U, Chiarolanza A, Fourrier-Reglat A, Moore N, Sturkemboom M, De Ponti F, Poluzzi E, Pariente A. Pharmacological prioritisation of signals of disproportionate reporting: proposal of an algorithm and pilot evaluation. Eur J Clin Pharmacol. 2014;70:617–25.
Poluzzi E, Raschi E, Koci A, Moretti U, Spina E, Behr ER, Sturkenboom M, De Ponti F. Antipsychotics and torsadogenic risk: signals emerging from the US FDA Adverse Event Reporting System database. Drug Saf. 2013;36:467–79.
Bate A, Pariente A, Hauben M, Bégaud B. Quantitative signal detection and analysis in pharmacovigilance. In: Andrews E, Moore N, editors. Mann’s Pharmacovigilance. London: Wiley; 2014. p. 331–54.
Poluzzi E, Raschi E, Motola D, Moretti U, De Ponti F. Antimicrobials and the risk of torsades de pointes: the contribution from data mining of the US FDA Adverse Event Reporting System. Drug Saf. 2010;33:303–14.
Roden DM. Taking the “idio” out of “idiosyncratic”: predicting torsades de pointes. Pacing Clin Electrophysiol. 1998;21:1029–34.
Raschi E, Poluzzi E, Koci A, Moretti U, Sturkenboom M, De Ponti F. Macrolides and torsadogenic risk: emerging issues from the FDA pharmacovigilance database. J Pharmacovigilance. 2013;1:104.
Raschi E, Poluzzi E, Godman B, Koci A, Moretti U, Kalaba M, Bennie M, Barbui C, Wettermark B, Sturkenboom M, De Ponti F. Torsadogenic risk of antipsychotics: combining adverse event reports with drug utilization data across Europe. PLoS One. 2013;8:e81208.
Poluzzi E, Raschi E, Godman B, Koci A, Moretti U, Kalaba M, Wettermark B, Sturkenboom M, De Ponti F. Pro-arrhythmic potential of oral antihistamines (H1): combining adverse event reports with drug utilization data across Europe. PLoS One. 2015;10:e0119551.
Wang HW, Hochberg AM, Pearson RK, Hauben M. An experimental investigation of masking in the US FDA adverse event reporting system database. Drug Saf. 2010;33:1117–33.
Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18:427–36.
Ahmed I, Thiessard F, Miremont-Salame G, Haramburu F, Kreft-Jais C, Begaud B, et al. Early detection of pharmacovigilance signals with automated methods based on false discovery rates: a comparative study. Drug Saf. 2012;35:495–506.
Harpaz R, DuMouchel W, LePendu P, Bauer-Mehren A, Ryan P, Shah NH. Performance of pharmacovigilance signal-detection algorithms for the FDA adverse event reporting system. Clin Pharmacol Ther. 2013;93:539–46.
Slattery J, Alvarez Y, Hidalgo A. Choosing thresholds for statistical signal detection with the proportional reporting ratio. Drug Saf. 2013;36:687–92.
Candore G, Juhlin K, Manlik K, Thakrar B, Quarcoo N, Seabroke S, Wisniewski A, Slattery J. Comparison of statistical signal detection methods within and across spontaneous reporting databases. Drug Saf. 2015;38:577–87.
Avillach P, Salvo F, Thiessard F, Miremont-Salamé G, Fourrier-Reglat A, Haramburu F, Bégaud B, Moore N, Pariente A, l’Association des Centres Régionaux de Pharmacovigilance. Pilot evaluation of an automated method to decrease false-positive signals induced by co-prescriptions in spontaneous reporting databases. Pharmacoepidemiol Drug Saf. 2014;23:186–94.
Grundmark B, Holmberg L, Garmo H, Zethelius B. Reducing the noise in signal detection of adverse drug reactions by standardizing the background: a pilot study on analyses of proportional reporting ratios-by-therapeutic area. Eur J Clin Pharmacol. 2014;70:627–35.
Poluzzi E, Raschi E, Piccinni C, De Ponti F. Data mining techniques in pharmacovigilance: analysis of the publicly accessible FDA Adverse Event Reporting System (AERS). In: Karahoca A, editor. Data mining applications in engineering and medicine. Croatia: Intech; 2012. p. 265–302.
Huber M, Kolzsch M, Rapp MA, Wulff I, Kalinowski S, Bolbrinker J, Hofmann W, Scholze J, Dräger D, Kreutz R. Antipsychotic drugs predominate in pharmacotherapy of nursing home residents with dementia. Pharmacopsychiatry. 2012;45:182–8.
Richter T, Mann E, Meyer G, Haastert B, Kopke S. Prevalence of psychotropic medication use among German and Austrian nursing home residents: a comparison of 3 cohorts. J Am Med Dir Assoc. 2012;13:187.
Sarganas G, Garbe E, Klimpel A, Hering RC, Bronder E, Haverkamp W. Epidemiology of symptomatic drug-induced long QT syndrome and Torsade de Pointes in Germany. Europace. 2014;16:101–8.
Huemer M, Boldt LH, Wutzler A, Parwani A, Rolf S, Blaschke D, Haverkamp W. Polymorphic ventricular tachycardia in a patient with herpes encephalitis. J Clin Neurosci. 2012;19:483–4.
Cohen AJ, Weiser B, Afzal Q, Fuhrer J. Ventricular tachycardia in two patients with AIDS receiving ganciclovir (DHPG). AIDS. 1990;4:807–9.
Hulhoven R, Rosillon D, Letiexhe M, Meeus MA, Daoust A, Stockis A. Levocetirizine does not prolong the QT/QTc interval in healthy subjects: results from a thorough QT study. Eur J Clin Pharmacol. 2007;63:1011–7.
Caillet C, Chauvelot-Moachon L, Montastruc JL, Bagheri H. Safety profile of enantiomers vs. racemic mixtures: it’s the same? Br J Clin Pharmacol. 2012;74:886–9.
Ravera S, Hummel SA, Stolk P, Heerdink RE, de Jong-van den Berg LT, de Gier JJ. The use of driving impairing medicines: a European survey. Eur J Clin Pharmacol. 2009;65:1139–47.
Italian Regulatory Agency. Nota Informativa Importante su Tinset (oxatomide). http://www.agenziafarmaco.gov.it/sites/default/files/nii_final_cts_settembre_10_0.pdf. Accessed 21 Apr 2015.
Iglesias E, Esteban E, Zabala S, Gascon A. Tiapride-induced torsade de pointes. Am J Med. 2000;109:509.
Jo SH, Lee SY. Response of i(kr) and HERG currents to the antipsychotics tiapride and sulpiride. Korean J Physiol Pharmacol. 2010;14:305–10.
Chong SA, Mythily, Lum A, Goh HY, Chan YH. Prolonged QTc intervals in medicated patients with schizophrenia. Hum Psychopharmacol. 2003;18:647–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007–2013) under grant agreement No. 241679—the ARITMO project.
Conflict of interest
Emanuel Raschi, Elisabetta Poluzzi, Francesco Salvo, Ariola Koci, Marc Suling, Stefania Antoniazzi, Luisella Perina, Lorna Hazell, Ugo Moretti, Antoine Pariente and Fabrizio De Ponti declare no conflict of interest relevant to the present manuscript. Miriam Sturkenboom is heading a research unit that holds unconditional research contracts with some pharmaceutical companies (EliLilly, Pfizer, AstraZeneca). None are related to this study. Edeltraut Garbe works for an institute that occasionally performs studies for some pharmaceutical companies (Bayer, Celgene, GSK, Mundipharma, Novartis, Sanofi-Aventis, Sanofi-Pasteur, Stada, Takeda). She has been a consultant for Bayer, GSK, Novartis, Schwabe, Takeda, and Teva. The present study is unrelated to these grants. The Drug Safety Research Unit has received unconditional grants from manufacturers of some of the drugs included in this study. None of these grants are related to this study.
Additional information
E. Raschi and E. Poluzzi equally contributed to the present work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Raschi, E., Poluzzi, E., Salvo, F. et al. The Contribution of National Spontaneous Reporting Systems to Detect Signals of Torsadogenicity: Issues Emerging from the ARITMO Project. Drug Saf 39, 59–68 (2016). https://doi.org/10.1007/s40264-015-0353-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-015-0353-1