Abstract
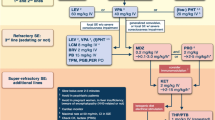
This article lays the background for, and discusses the practical issues surrounding, the adjunctive use of the last four antiepileptic drugs (AEDs) to be licensed for the treatment of pharmacoresistant focal seizures in the UK and elsewhere. More than 30 % of adolescent and adult patients will not be fully controlled on the currently available therapeutic armamentarium. After not responding to their first three AED schedules, only a handful of patients attained seizure freedom on subsequent regimens. To optimise the response to any new AED in this setting, it is often necessary to reduce the existing drug burden. The pharmacology, tolerability and safety, and everyday use of lacosamide, eslicarbazepine acetate, retigabine (ezogabine) and perampanel will be reviewed and discussed. This will be accompanied by data from prospective audits with each drug undertaken at the Western Infirmary in Glasgow, Scotland, and a report of their successful introduction in an illustrative case. Overall, there is a large variation in the course of refractory epilepsy and the effect of AED therapy on this process seems minimal. Nevertheless, a number of patients will benefit from the introduction of each new AED, with some becoming seizure-free.




Similar content being viewed by others
References
Lőscher W, Klitgaard H, Twyman RE, Schmidt D. New avenues for antiepileptic drug discovery and development. Nat Rev Drug Discov. 2013;12:757–76.
Brodie MJ, Covanis A, Lerche H, Perucca E, Sills GJ, White S. Antiepileptic drug therapy: does mechanism of action matter? Epilepsy Behav. 2011;21:331–41.
Brodie MJ. Road to refractory epilepsy: the Glasgow story. Epilepsia. 2013;54(Suppl 2):5–8.
Chen Z, Brodie MJ, Liew D, Kwan P. Long-term outcome of 1805 people with newly diagnosed epilepsy. In: Presented at the 31st international epilepsy congress, 5–9 Sep 2015; Istanbul.
Lőscher W, Schmidt D. Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia. 2011;52:657–78.
Brodie MJ, Barry SJE, Bamagous GA, Norrie J, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78:1548–54.
Brodie MJ, Kelly K, Stephen LJ. Prospective audits with new antiepileptic drugs: insights into response. Epilepsy Behav. 2014;31:73–6.
Brodie MJ, Sills GJ. Combining antiepileptic drugs: rational polytherapy. Seizure. 2011;20:369–75.
Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Hauser WA, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–77.
Hao X, Goldberg D, Kelly K, Stephen L, Kwan P, Brodie MJ. Uncontrolled epilepsy is not necessarily the same as drug-resistant epilepsy. Differences between populations with newly diagnosed and chronic epilepsy. Epilepsy Behav. 2013;29:4–6.
Luoni C, Bisulli F, Canevini MP, De Sarro G, Fattore C, Galimberti CA, On behalf of the SOPHIE Study Group, et al. Determinants of health-related quality of life in pharmacoresistant epilepsy: results from a large multicenter study of consecutively enrolled patients using validated quantitative assessments. Epilepsia. 2011;52:2181–91.
Stephen LJ, Forsyth M, Kelly K, Brodie MJ. Antiepileptic drug combinations: have newer agents altered clinical outcomes? Epilepsy Res. 2012;98:194–8.
Weschler RT, Li G, French J, O’Brien TJ, D’Cruz O, Williams P, On behalf of the ALEX-MT Study Group, et al. Conversion to lacosamide monotherapy in the treatment of focal epilepsy: results from a historically-controlled, multicentre double blind study. Epilepsia. 2014;55:1088–98.
Ben-Menachem E, Biton V, Jatuzis D, Abou-Khalil B, Doty P, Rudd GD. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia. 2007;48:1308–17.
Halasz P, Kalvianen R, Mazurkiewicz-Beldzinska M, Rosenow F, Doty P, Herbert D, Sullivan T, On behalf of the SP755 Study Group. Adjunctive lacosamide for partial-onset seizures: efficacy and safety results from a randomized controlled trial. Epilepsia. 2009;50:443–53.
Chung S, Sperling MR, Biton V, Krauss G, Herbert D, Rudd GB, On behalf of the SP754 Study Group, et al. Lacosamide as adjunctive therapy for partial-onset seizures? A randomized controlled trial. Epilepsia. 2010;51:958–67.
Stephen J, Brodie MJ. Pharmacotherapy of epilepsy. Newly approved and developmental agents. CNS Drugs. 2011;25:89–107.
Errington AC, Stőhr T, Heers C, Lees G. The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage-gated sodium channels. Mol Pharmacol. 2008;73:157–69.
Saké J-K, Hebert D, Isojarvi J, Doty P, De Backer M, Davies SK, et al. A pooled analysis of lacosamide clinical trial grouped by mechanisms of action of concomitant antiepileptic drugs. CNS Drugs. 2010;24:1055–8.
Cross SA, Curran MP. Lacosamide in partial-onset seizures. Drugs. 2009;69:449–59.
Canello W, Rosencranz B, Schmid B, Wierich W. Pharmacodynamic and pharmacokinetic evaluation of co-administration of lacosamide and an oral contraceptive (levonorgestrel plus ethinyloestradiol) in healthy female volunteers. Epilepsia. 2013;54:530–6.
Zaccara G, Perucca P, Loiacono G, Giovannelli F, Verrotti A. The adverse event profile of lacosamide: A systematic review and meta-analysis of randomized controlled trials. Epilepsia. 2013;54:66–74.
Stephen LJ, Kelly K, Parker P, Brodie MJ. Adjunctive lacosamide: 5 years clinical experience. Epilepsy Res. 2014;108:1385–91.
De Giorgio CM. Atrial flutter/atrial fibrillation associated with lacosamide for partial seizures. Epilepsy Behav. 2010;18:322–4.
Nizam A, Mylayarapu K, Thomas D, Briskin K, Wu B, Saluja D, et al. Lacosamide-induced second-degree atrioventricular block in a patient with partial epilepsy. Epilepsia. 2011;52:e153–5.
Villanueva V, Lopez-Gomariz E, Lopez-Trigo J, Palau J, Garcia M, Villaroya T, et al. Rational polytherapy with lacosamide in clinical practice: results of a Spanish cohort analysis RELACOVA. Epilepsy Behav. 2012;23:298–304.
Flores L, Kemp S, Colbeck K, Moran N, Quirk J, Ramkolea P, et al. Clinical experience with oral lacosamide as adjunctive therapy in adult patients with uncontrolled epilepsy: a multicentre study in epilepsy clinics in the United Kingdom. Seizure. 2012;21:512–7.
Novy J, Bartolini E, Bell GS, Duncan JS, Sander JW. Long-term retention of lacosamide in a large cohort of people with medically refractory epilepsy: a single centre evaluation. Epilepsy Res. 2013;106:250–6.
Villanueva V, Lopez FJ, Serratosa JM, Gonzalez-Giraldex B, Campos D, Molins A, et al. Control of seizures in different stages of partial epilepsy: LACO-EXP, a Spanish retrospective study of lacosamide. Epilepsy Behav. 2013;29:349–56.
Paquette V, Culley C, Greanya ED, Ensom MHH. Lacosamide as adjunctive therapy in refractory epilepsy in adults: a systematic review. Seizure. 2015;25:1–17.
Zangaladze A, Skidmore C. Lacosamide use in refractory idiopathic primary generalised epilepsy. Epilepsy Behav. 2012;23:79–80.
Afra P, Adamolekun B. Lacosamide treatment of juvenile myoclonic epilepsy. Seizure. 2012;21:202–4.
Hőfler J, Unterberger I, Dobesberger J, Kuchukhiaze G, Walser G, Trinka E. Intravenous lacosamide in status epilepticus and seizure clusters. Epilepsia. 2011;53:e148–52.
Kellinghaus C, Berning S, Immisch I, Larch J, Rosenaw F, Rossetti AO, et al. Intravenous lacosamide for treatment of status epilepticus. Acta Neurol Scand. 2011;123:137–41.
Koubeissi MZ, Mayor CL, Estaphan B, Rashid S, Azar JN. Efficacy and safety of intravenous lacosamide in refractory nonconvulsive status epilepticus. Acta Neurol Scand. 2011;123:142–6.
Stephen LJ, Kelly K, Parker P, Brodie MJ. Adjunctive lacosamide in clinical practice-sodium blockade with a difference? Epilepsy Behav. 2011;22:499–504.
Hitiris N, Mohanraj R, Norrie J, Sills GJ, Brodie MJ. Predictors of pharmacoresistant epilepsy. Epilepsy Res. 2007;75:192–6.
Gil-Nagel A, Elger C, Ben-Menachem E, Halasz P, Lopes-Lima J, Gabbai AA, et al. Efficacy and safety of eslicarbazepine acetate as add-on treatment in patients with focal seizures: integrated analysis of pooled data from double-blind phase III clinical studies. Epilepsia. 2013;54:98–107.
Bialer M, Soares-da-Silva P. Pharmacokinetics and drug interactions of eslicarbazepine acetate. Epilepsia. 2012;53:939–46.
Keating GM. Eslicarbazepine acetate: a review of its use as adjunctive therapy in refractory partial-onset seizures. CNS Drugs. 2014;28:583–600.
Hebeisen S, Pires N, Loureiro AI, Bonifacio MJ, Palma N, Whyment A, et al. Eslicarbazepine and the enhancement of slow inactivation of voltage-gated sodium channels: a comparison with carbamazepine, oxcarbazepine and lacosamide. Neuropharmacology. 2015;89:122–35.
Falĉao A, Vaz-da-Silva M, Gama H, Nunes T, Almeida L, Soares-da-Silva P. Effect of eslicarbazepine acetate on the pharmacokinetics of a combined ethinyloestradiol/levonorgestrel oral contraceptive in healthy women. Epilepsy Res. 2013;105:368–76.
Gupta DK, Bhoi SK, Kalita J, Misra UK. Hyponatraemia following eslicarbazepine therapy. Seizure. 2015;29:11–4.
Chung SS. Third generation antiepileptic drugs for partial-onset seizures: lacosamide, retigabine and eslicarbazepine acetate. Eur Neurol J. 2009;1:1–11.
Hufnagel A, Ben-Menachem E, Gabbai AA, Falcao A, Almeida L, Soares-da-Silva P. Long-term safety and efficacy of eslicarbazepine acetate as adjunctive therapy in the treatment of partial-onset seizures in adults with epilepsy: results of a 1-year open-label extension study. Epilepsy Res. 2013;103:262–9.
Villaneuva V, Serratosa JM, Guillamone E, Garces M, Giraldez BG, Toledo M, et al. Long-term safety and efficacy of eslicarbazepine acetate in patients with focal seizures: results of a 1-year ESLIBASE retrospective study. Epilepsy Res. 2014;108:1243–52.
Massot A, Vivanco R, Principe A, Roquer J, Rocamora R. Post-authorisation study of eslicarbazepine as treatment for drug-resistant epilepsy: preliminary results. Neurologia. 2014;29:94–10.
Porter RJ, Partiot A, Sachdeo R, Nohria V, Alves WM, For the 205 Study Group. Randomized, multicenter dose-ranging trial of retigabine for partial-onset seizures. Neurology. 2007;68:1197–204.
Brodie MJ, Lerche H, Gil-Nagel A, Elger C, Hall, S, Shin P, On behalf of the RESTORE 2 Study Group, et al. Efficacy and safety of adjunctive retigabine/ezogabine in refractory partial epilepsy. Neurology. 2010;75:1817–24.
French JA, Abou-Khalil BW, Leroy R, Yacubian EM, Shin P, Hall S, On behalf of the RESTORE 1 Study 301 investigators, et al. Randomized, double-blind, placebo-controlled trial of ezgogabine (retigabine) in partial epilepsy. Neurology. 2011;76:1555–63.
Wuttke TV, Seebohn G, Bail S, Maljevic S, Lerche H. The new anticonvulsant retigabine favours voltage-dependent opening of the Kv 7.2 (KCNQ2) channel by binding to its activation gate. Mol Pharmacol. 2005;67:1009–17.
Gunthorpe MJ, Large CH, Sankar R. The mechanism of action of retigabine (ezogabine), a first in class K+ channel opener for the treatment of epilepsy. Epilepsia. 2012;53:412–24.
Treven M, Koenig X, Assadpour E, Gantumur E, Meyer C, Hilber K, et al. The anticonvulsant retigabine is a subtype selective modulation of GABAA receptors. Epilepsia. 2015;56:647–57.
Brodie MJ, Mintzer S, Pack AM, Gidal BE, Vecht CJ, Schmidt D. Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia. 2013;54:11–27.
Ferron GM, Patat A, Parks V, Nolan P, Troy SM. Lack of pharmacokinetic interaction between retigabine and phenobarbitone at steady state in healthy subjects. Br J Clin Pharmacol. 2003;56:39–45.
Hermann R, Knebel NG, Niebch G, Richards L, Borlak J, Locher M. Pharmacokinetic interaction between retigabine and lamotrigine in healthy subjects. Eur J Clin Pharmacol. 2003;58:795–802.
Brickell N, Gandhi P, van Landingham K, Hammond J, De Rossett S. The urinary safety profile and secondary renal effects of retigabine (ezogabine): a first-in-class antiepileptic drug that targets KCNQ (Kv7) potassium channels. Epilepsia. 2012;53:606–12.
Gil-Nagel A, Brodie MJ, Leroy R, Cyr T, Hall S, Castiglia M, et al. Safety and efficacy of ezogabine (retigabine) in adults with refractory partial-onset seizures: interim results from two ongoing open-label studies. Epilepsy Res. 2012;102:117–21.
Shkolnik TG, Feuerman H, Didkovsky E, Kaplan I, Bergman R, Pavlovsky L, et al. Blue-gray mucocutaneous discolouration-a new adverse effect of ezogabine. JAMA Dermatol. 2014;150:984–9.
Evans SM, Akintayo O, Harrington CM, Kelly DB, Mcdonald SA, Lee W-J, et al. Pigmentation abnormalities (discolorations) associated with ezogabine/retigabine treatment: clinical effects. In: Presented at the 68th annual american epilepsy society meeting, 6–9 Dec 2014: Seattle (WA).
Beacher NG, Brodie MJ, Goodall C. A case report: retigabine induced oral mucosal dyspigmentation of the hard palate. BMC Oral Health. 2015;15(1):122.
Prescott JS, Evans GA. Pigmentary abnormalites (discoloration) associated with ezogabine/retigabine treatment: non clinical aspects. In: Presented at the 68th annual american epilepsy society meeting, 6–9 Dec 2014: Seattle (WA).
Wehner T, Chinnasami S, Novy J, Bell GS, Duncan JS, Sander JW. Long term retention of retigabine in a cohort of people with drug resistant epilepsy. Seizure. 2014;23:876–81.
Huber B, Bocchiccio M. A retrospective evaluation of retigabine in patients with highly therapy-resistant epilepsy. Epilepsy Behav. 2015;44:234–7.
Lerche H, Daniluk J, Lotay N, De Rossett S, Edwards S, Brandt C. Efficacy and safety of ezogabine/retigabine as adjunctive therapy to specified single antiepileptic medications in an open-label study of adults with partial-onset seizures. Seizure. 2015;30:93–100.
Steinhoff BJ, Ben-Menachem E, Ryvlin P, Shorvon S, Kramer L, Satlin A, et al. Efficacy and safety of adjunctive perampanel for the treatment of refractory partial seizures: a pooled analysis of three phase III studies. Epilepsia. 2013;54:1481–9.
Rogawski MA. Revisiting AMPA receptors as an antiepileptic drug target. Epilepsy Curr. 2011;11:56–63.
Patsalos P. The clinical pharmacology profile of the new antiepileptic drug perampanel: a novel non competitive AMPA receptor antagonist. Epilepsia. 2015;56:12–27.
Gidal BE, Ferry J, Majid O, Hussein Z. Concentration-effect relationships with perampanel in patients with pharmacoresistant partial-onset seizures. Epilepsia. 2013;54:1490–7.
Plosker GL. Perampanel as adjunctive therapy in patients with partial-onset seizures. CNS Drugs. 2012;26:1085–96.
Frampton JE. Perampanel: a review in drug-resistant epilepsy. Drugs. 2015;75(14):1657–68. doi:10.1007/s40265-015-0465-z.
Zaccara G, Giovanelli F, Cincotta M, Verrotti A, Grillo E. The adverse event profile of perampanel: meta-analysis of randomized controlled trials. Eur J Neurol. 2013;20:1204–11.
Yang H, Laurenza A, Williams B, Patten A, Hussein Z, Ferry J. Lack of effect of perampanel on QT interval duration: results from a thorough QT analysis and pooled partial seizure phase III clinical trials. Epilepsy Res. 2015;114:122–30.
Ettinger AB, Lopresti A, Yang H, Williams B, Zhou S, Fain R, et al. Psychiatric and behavioural adverse events in randomized clinical studies of the non-competitive AMPA receptor antagonist perampanel. Epilepsia. 2015;56:1252–63.
Rosenfeld W, Conry J, Lagae L, Rozentals G, Yang H, Fain R, et al. Efficacy and safety of perampanel in adolescent patients with drug-resistant partial seizures in three double-blind, placebo-controlled phase III randomized clinical studies and a combined extension study. Eur J Paediatr Neurol. 2015;19:435–45.
Coyle H, Clough P, Cooper P, Mohanraj R. Clinical experience with perampanel: focus on psychiatric adverse effects. Epilepsy Behav. 2014;41:193–6.
Huber B. Increased risk of suicidality on perampanel (Fycompa)? Epilepsy Behav. 2014;31:71–2.
Steinhoff BJ, Hamer H, Trinka E, Schulze-Bonhage A, Bien C, Mayer T, et al. A multicentre survey of clinical experience with perampanel in real life in Germany and Austria. Epilepsy Res. 2014;1058:986–8.
Gidal BE, Laurenza A, Hussein Z, Yang H, Fain R, Edelstein J, et al. Perampanel efficacy and tolerability with enzyme-inducing AEDs in patients with epilepsy. Neurology. 2015;84:1–9.
Kramer LD, Satlin A, Krauss GL, French J, Perucca E, Ben-Menachem E, et al. Perampanel for adjunctive treatment of partial-onset seizures: a pooled dose-response analysis of phase III studies. Epilepsia. 2014;55:423–43.
French JA, Krauss G, Wechsler R, Wang X, Diventura B, Brandt C, et al. Adjunctive perampanel for treatment of drug-resistant primary generalised tonic-clonic seizures in patients with idiopathic generalised epilepsy: a double-blind randomized placebo-controlled phase III trial. Neurology. 2015;85:950–7.
Piedad J, Rickards H, Besag FG, Cavana AE. Benefical and adverse psychiatric effects of antiepileptic drugs in patients with epilepsy. CNS Drugs. 2012;26:319–35.
Lin JL, Mula M, Hermann BP. Uncovering the neurobehavioural comorbidities of epilepsy over a lifespan. Lancet. 2012;380:1180–92.
Geerts A, Brouwer O, Stroink H, Van Donselaar C, Peters B, Peeters G, et al. Onset of intractability and its course and time: the Dutch study of epilepsy in children. Epilepsia. 2012;53:741–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received in support of the publication of this article.
Conflicts of interest
Martin Brodie serves on the Scientific Advisory Boards of Eisai Ltd, UCB Pharma, GlaxoSmithKline, Lundbeck, Bial, GW Pharmaceuticals and Takeda. He is on the speakers’ bureau for Eisai Ltd, UCB Pharma, GlaxoSmithKline and Lundbeck, and has accepted travel grants for scientific meetings from Eisai Ltd, UCB Pharma and Lundbeck.
Rights and permissions
About this article
Cite this article
Brodie, M.J. Practical Use of Newer Antiepileptic Drugs as Adjunctive Therapy in Focal Epilepsy. CNS Drugs 29, 893–904 (2015). https://doi.org/10.1007/s40263-015-0285-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-015-0285-4