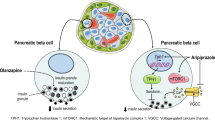
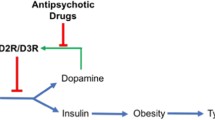
Abstract
Second generation antipsychotics (SGAs) are widely prescribed to treat various disorders, most notably schizophrenia and bipolar disorder; however, SGAs can cause abnormal glucose metabolism that can lead to insulin-resistance and type 2 diabetes mellitus side-effects by largely unknown mechanisms. This review explores the potential candidature of the acetylcholine (ACh) muscarinic M3 receptor (M3R) as a prime mechanistic and possible therapeutic target of interest in SGA-induced insulin dysregulation. Studies have identified that SGA binding affinity to the M3R is a predictor of diabetes risk; indeed, olanzapine and clozapine, SGAs with the highest clinical incidence of diabetes side-effects, are potent M3R antagonists. Pancreatic M3Rs regulate the glucose-stimulated cholinergic pathway of insulin secretion; their activation on β-cells stimulates insulin secretion, while M3R blockade decreases insulin secretion. Genetic modification of M3Rs causes robust alterations in insulin levels and glucose tolerance in mice. Olanzapine alters M3R density in discrete nuclei of the hypothalamus and caudal brainstem, regions that regulate glucose homeostasis and insulin secretion through vagal innervation of the pancreas. Furthermore, studies have demonstrated a dynamic sensitivity of hypothalamic and brainstem M3Rs to altered glucometabolic status of the body. Therefore, the M3R is in a prime position to influence glucose homeostasis through direct effects on pancreatic β-cells and by potentially altering signalling in the hypothalamus and brainstem. SGA-induced insulin dysregulation may be partly due to blockade of central and peripheral M3Rs, causing an initial disruption to insulin secretion and glucose homeostasis that can progressively lead to insulin resistance and diabetes during chronic treatment.

Similar content being viewed by others
References
Ballard C, Waite J, Birks J. Atypical antipsychotics for aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006. 2006(Issue 1):Art. No.: CD003476. doi:10.1002/14651858.CD003476.pub2.
Brambilla F, Monteleone P, Maj M. Olanzapine-induced weight gain in anorexia nervosa: Involvement of leptin and ghrelin secretion? Psychoneuroendocrinology. 2007;32(4):402–6.
Bridle C, Palmer S, Bagnall AM, Darba J, Duffy S, Sculpher M, et al. A rapid and systematic review and economic evaluation of the clinical and cost-effectiveness of newer drugs for treatment of mania associated with bipolar affective disorder. Health Technol Assess. 2004;8(19):iii–iv.
Budman C, Gayer A, Lesser M, Shi Q, Bruun R. An open-label study of the treatment efficacy of olanzapine for Tourette’s disorder. J Clin Psychiatry. 2001;62(4):290–4.
Centorrino F, Eakin M, Bahk W-M, Kelleher JP, Goren J, Salvatore P, et al. Inpatient Antipsychotic Drug Use in 1998, 1993, and 1989. Am J Psychiatry. 2002;159(11):1932–5.
De Hert M, Dobbelaere M, Sheridan EM, Cohen D, Correll CU. Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: a systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur Psychiatry. 2011;26(3):144–58.
Frenchman IB. Atypical antipsychotics for nursing home patients: a retrospective chart review. Drugs Aging. 2005;22(3):257–64.
Pickar D, Vinik J, Bartko JJ. Pharmacotherapy of schizophrenic patients: preponderance of off-label drug use. PLoS One. 2008;3(9):e3150.
Zuddas A, Zanni R, Usala T. Second generation antipsychotics (SGAs) for non-psychotic disorders in children and adolescents: a review of the randomized controlled studies. Eur Neuropsychopharmacol. 2011;21(8):600–20.
Khojainova N, Santiago-Palma J, Kornick C, Breitbart W, Gonzales GR. Olanzapine in the management of cancer pain. J Pain Symptom Manage. 2002;23(4):346–50.
Elsayem A, Bush SH, Munsell MF, Curry Iii E, Calderon BB, Paraskevopoulos T, et al. Subcutaneous olanzapine for hyperactive or mixed delirium in patients with advanced cancer: a preliminary study. J Pain Symptom Manage. 2010;40(5):774–82.
Baptista T. Body weight gain induced by antipsychotic drugs: mechanisms and management. Acta Psychiatr Scand. 1999;100(1):3–16.
Newcomer J. Second-generation atypical antipsychotics and metabolic effects. A comprehensive literature review. CNS Drugs. 2005;19:1–93.
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. The clinical antipsychotic trials of intervention effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–23.
Rader DJ. Effect of insulin resistance, dyslipidemia, and Intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med. 2007;120(3, Suppl. 1):S12–8.
Stahl SM, Mignon L, Meyer JM. Which comes first: atypical antipsychotic treatment or cardiometabolic risk? Acta Psychiatr Scand. 2009;119(3):171–9.
American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, Obesity NAAftSo. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596–601.
Oriot P, Feys J, Mertens de Wilmars S, Misson S, Ayache A, Fagnart O, et al. Insulin sensitivity, adjusted beta-cell function and adiponectinaemia among lean drug-naive schizophrenic patients treated with atypical antipsychotic drugs: a nine-month prospective study. Diabetes Metab. 2008;34(5):490–6.
Lambert MT, Copeland LA, Sampson N, Duffy SA. New-onset type-2 diabetes associated with atypical antipsychotic medications. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(5):919–23.
Perez-Iglesias R, Vazquez-Barquero JL, Amado JA, Berja A, Garcia-Unzueta MT, Pelayo-Teran JM, et al. Effect of antipsychotics on peptides involved in energy balance in drug-naive psychotic patients after 1 year of treatment. J Clin Psychopharmacol. 2008;28(3):289–95.
Sacher J, Mossaheb N, Spindelegger C, Klein N, Geiss-Granadia T, Sauermann R, et al. Effects of olanzapine and ziprasidone on glucose tolerance in healthy volunteers. Neuropsychopharmacology. 2008;33(7):1633–41.
Wu R, Zhao J, Guo X, He Y, Fang M, Guo W, et al. Metformin addition attenuates olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients: a double-blind, placebo-controlled study. Am J Psychiatry. 2008;165(3):352–8.
Albaugh VL, Judson JG, She P, Lang CH, Maresca KP, Joyal JL, et al. Olanzapine promotes fat accumulation in male rats by decreasing physical activity, repartitioning energy and increasing adipose tissue lipogenesis while impairing lipolysis. Mol Psychiatry. 2010;16(5):569–81.
Shobo M, Yamada H, Mihara T, Kondo Y, Irie M, Harada K, et al. Two models for weight gain and hyperphagia as side effects of atypical antipsychotics in male rats: validation with olanzapine and ziprasidone. Behav Brain Res. 2010;216(2):561–8.
Boyda HN, Tse L, Procyshyn RM, Wong D, Wu TKY, Pang CC, et al. A parametric study of the acute effects of antipsychotic drugs on glucose sensitivity in an animal model. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):945–54.
Chintoh AF, Mann SW, Lam L, Giacca A, Fletcher P, Nobrega J, et al. Insulin resistance and secretion in vivo: effects of different antipsychotics in an animal model. Schizophr Res. 2009;108(1–3):127–33.
Coccurello R, Brina D, Caprioli A, Conti R, Ghirardi O, Schepis F, et al. 30 days of continuous olanzapine infusion determines energy imbalance, glucose intolerance, insulin resistance, and dyslipidemia in mice. J Clin Psychopharmacol. 2009;29(6):576–83.
Park S, Hong S, Ahn I, Kim D, Kim S. Estrogen replacement reverses olanzapine-induced weight gain and hepatic insulin resistance in ovariectomized diabetic rats. Neuropsychobiology. 2010;61(3):148.
van der Zwaal EM, Luijendijk MCM, Evers SS, la Fleur SE, Adan RAH. Olanzapine affects locomotor activity and meal size in male rats. Pharmacol Biochem Behav. 2010;97(1):130–7.
Weston-Green K, Huang X-F, Deng C. Olanzapine treatment and metabolic dysfunction: a dose response study in female Sprague Dawley rats. Behav Brain Res. 2011;217(2):337–46.
Tulipano G, Rizzetti C, Bianchi I, Fanzani A, Spano P, Cocchi D. Clozapine-induced alteration of glucose homeostasis in the rat: the contribution of hypothalamic-pituitary-adrenal axis activation. Neuroendocrinology. 2007;85(2):61.
Boyda HN, Tse L, Procyshyn RM, Honer WG, Barr AM. Preclinical models of antipsychotic drug-induced metabolic side effects. Trends Pharmacol Sci. 2010;31(10):484–97.
Coccurello R, Moles A. Potential mechanisms of atypical antipsychotic-induced metabolic derangement: clues for understanding obesity and novel drug design. Pharmacol Ther. 2010;127(3):210–51.
Houseknecht KL, Robertson AS, Zavadoski W, Gibbs EM, Johnson DE, Rollema H. Acute effects of atypical antipsychotics on whole-body insulin resistance in rats: implications for adverse metabolic effects. Neuropsychopharmacology. 2006;32(2):289–97.
Chintoh AF, Mann SW, Lam L, Lam C, Cohn TA, Fletcher PJ, et al. Insulin resistance and decreased glucose-stimulated insulin secretion after acute olanzapine administration. J Clin Psychopharmacol. 2008;28(5):494–9.
Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med. 2001;111(9):716–23.
Strassnig M, Awerbuck J, Ganguli R. Diabetes resolution following discontinuation of a second-generation antipsychotic: three cases. Clin Schizophrenia Relat Psychoses. 2012;6(4):202–3.
Boyda HN, Procyshyn RM, Tse L, Wong D, Pang CC, Honer WG, et al. Intermittent treatment with olanzapine causes sensitization of the metabolic side-effects in rats. Neuropharmacology. 2012;62(3):1391–400.
Johnson DE, Yamazaki H, Ward KM, Schmidt AW, Lebel WS, Treadway JL, et al. Inhibitory effects of antipsychotics on carbachol-enhanced insulin secretion from perifused rat islets: role of muscarinic antagonism in antipsychotic-induced diabetes and hyperglycemia. Diabetes. 2005;54(5):1552–8.
Chiu C, Chen K, Liu H, Lu M. The early effect of olanzapine and risperidone on insulin secretion in atypical-naive schizophrenic patients. J Clin Psychopharmacol. 2006;26(5):504–7.
Chiu C-C, Chen C-H, Chen B-Y, Yu S-H, Lu M-L. The time-dependent change of insulin secretion in schizophrenic patients treated with olanzapine. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):866–70.
Sowell MO, Mukhopadhyay N, Cavazzoni P, Shankar S, Steinberg HO, Breier A, et al. Hyperglycemic clamp assessment of insulin secretory responses in normal subjects treated with olanzapine, risperidone, or placebo. J Clin Endocrinol Metab. 2002;87(6):2918–23.
Hardy TA, Meyers AL, Yu J, Shankar SS, Steinberg HO, Porksen NK. Acute insulin response and β-cell compensation in normal subjects treated with olanzapine or risperidone for 2 weeks. Diabetes Care. 2007;30(1):157–8.
Ader M, Kim SP, Catalano KJ, Ionut V, Hucking K, Richey JM, et al. Metabolic dysregulation with atypical antipsychotics occurs in the absence of underlying disease a placebo-controlled study of olanzapine and risperidone in dogs. Diabetes. 2005;54(3):862–71.
Albaugh VL, Singareddy R, Mauger D, Lynch CJ. A double blind, placebo-controlled, randomized crossover study of the acute metabolic effects of olanzapine in healthy volunteers. PLoS One. 2011;6(8):e22662.
Teff KL, Rickels MR, Grudziak J, Fuller C, Nguyen HL, Rickels K. Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes. 2013;62(9):3232–40.
Henderson JR, Jefferys DB, Jones RH, Stanley D. The effect of atropine on the insulin release caused by oral and intravenous glucose in human subjects. Acta Endocrinol (Cph). 1976;83(4):772–80.
Ohnuma H, Yamatani K, Igarashi M, Sugiyama K, Manaka H, Tominaga M, Sasaki H. Intracerebroventricular injection of methylatropine suppresses insulin-response to oral glucose-load in rats. J Auton Nerv Syst. 1996;57(1–2):43–8.
Ahren B, Pacini G. Importance of quantifying insulin secretion in relation to insulin sensitivity to accurately assess beta cell function in clinical studies. Eur J Endocrinol. 2004;150(2):97–104.
Uwaifo GI, Parikh SJ, Keil M, Elberg J, Chin J, Yanovski JA. Comparison of insulin sensitivity, clearance, and secretion estimates using euglycemic and hyperglycemic clamps in children. J Clin Endocrinol Metab. 2002;87(6):2899–905.
Melkersson K. Clozapine and olanzapine, but not conventional antipsychotics, increase insulin release in vitro. Eur Neuropsychopharmacol. 2004;14(2):115–9.
Sasaki N, Iwase M, Uchizono Y, Nakamura U, Imoto H, Abe S, et al. The atypical antipsychotic clozapine impairs insulin secretion by inhibiting glucose metabolism and distal steps in rat pancreatic islets. Diabetologia. 2006;49(12):2930–8.
Smith G, Chaussade C, Vickers M, Jensen J, Shepherd P. Atypical antipsychotic drugs induce derangements in glucose homeostasis by acutely increasing glucagon secretion and hepatic glucose output in the rat. Diabetologia. 2008;51(12):2309–17.
Manu P, Correll CU, Wampers M, van Winkel R, Yu W, Shiffeldrim D, et al. Insulin secretion in patients receiving clozapine, olanzapine, quetiapine and risperidone. Schizophr Res. 2013;143(2–3):358–62.
Bouche C, Serdy S, Kahn CR, Goldfine AB. The cellular fate of glucose and its relevance in type 2 diabetes. Endocr Rev. 2004;25(5):807–30.
Thorens B. Central control of glucose homeostasis: the brain—endocrine pancreas axis. Diabetes Metab. 2010;36(Suppl. 3):S45–9.
Knauf C, Cani PD, Kim D-H, Iglesias MA, Chabo C, Waget A, et al. Role of central nervous system glucagon-like peptide-1 receptors in enteric glucose sensing. Diabetes. 2008;57(10):2603–12.
Gilon P, Henquin J-C. Mechanisms and physiological significance of the cholinergic control of pancreatic {beta}-cell function. Endocr Rev. 2001;22(5):565–604.
Ahrén B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia. 2000;43(4):393–410.
Osundiji MA, Evans ML. Brain control of insulin and glucagon secretion. Endocrinol Metab Clin North Am. 2013;42(1):1–14.
Burcelin R. The gut-brain axis: a major glucoregulatory player. Diabetes Metab. 2010;36(Suppl. 3):S54–8.
Ahren B, Pacini G. Dose-related effects of GLP-1 on insulin secretion, insulin sensitivity, and glucose effectiveness in mice. Am J Physiol Endocrinol Metab. 1999;277(6):E996–1004.
Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–57.
Fujiwara K, Gotoh K, Chiba S, Masaki T, Katsuragi I, Kakuma T, et al. Intraportal administration of DPP-IV inhibitor regulates insulin secretion and food intake mediated by the hepatic vagal afferent nerve in rats. J Neurochem. 2012;121(1):66–76.
Blake CB, Smith BN. Insulin reduces excitation in gastric-related neurons of the dorsal motor nucleus of the vagus. Am J Physiol Regul Integr Comp Physiol. 2012;303(8):R807–14.
Pardini AW, Nguyen HT, Figlewicz DP, Baskin DG, Williams DL, Kim F, et al. Distribution of insulin receptor substrate-2 in brain areas involved in energy homeostasis. Brain Res. 2006;1112(1):169–78.
Unger JW, Moss AM, Livingston JN. Immunohistochemical localization of insulin receptors and phosphotyrosine in the brainstem of the adult rat. Neuroscience. 1991;42(3):853–61.
Kalra SP. Disruption in the leptin-NPY link underlies the pandemic of diabetes and metabolic syndrome: New therapeutic approaches. Nutrition. 2008;24(9):820–6.
Schwartz MW, Porte D Jr. Diabetes, obesity, and the brain. Science. 2005;307(5708):375–9.
Konturek SJ, Zabielski R, Konturek JW, Czarnecki J. Neuroendocrinology of the pancreas; role of brain-gut axis in pancreatic secretion. Eur J Pharmacol. 2003;481(1):1–14.
Pavlov I. Lectures on conditioned reflexes. 3rd ed. London: Lawrence and Wishart; 1941.
Frohman LA, Bernardis LL, Schnatz JD, Burek L. Plasma insulin and triglyceride levels after hypothalamic lesions in weanling rats. Am J Physiol. 1969;216:1496–501.
Hales CN, Kennedy GC. Plasma glucose, nonesterified fatty acid and insulin concentrations in hypothalamic-hyperphagia rats. Biochem J. 1964;90:620–4.
Rohner-Jeanrenaud F, Jeanrenaud B. Consequences of ventromedial hypothalamic lesions upon insulin and glucagon secretion by subsequently isolated perfused pancreases in the rat. J Clin Invest. 1980;65:902–10.
Berthoud H-R, Jeanrenaud B. Acute hyperinsulinemia and its reversal by vagotomy after lesions of the ventromedial hypothalamus in anesthetized rats. Endocrinology. 1979;105:146–51.
Bereiter DA, Rohner-Jeanrenaud F, Berthoud H-R, Jeanrenaud B. CNS modulation of pancreatic endocrine function. Diabetologia. 1981;20:417–25.
Guillod-Maximin E, Lorsignol A, Alquier T, Pénicaud L. Acute intracarotid glucose injection towards the brain induces specific c-fos activation in hypothalamic nuclei: involvement of astrocytes in cerebral glucose-sensing in rats. J Neuroendocrinol. 2004;16(5):464–71.
Yuan P-Q, Yang H. Neuronal activation of brain vagal-regulatory pathways and upper gut enteric plexuses by insulin hypoglycemia. Am J Physiol Endocrinol Metab. 2002;283(3):E436–48.
Elmquist JK, Marcus JN. Rethinking the central causes of diabetes. Nat Med. 2003;9(6):645–7.
Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med. 2003;9:756–61.
Obici S, Feng Z, Karkanias G, Baskin D, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5(6):566–72.
Schwartz MW. Progress in the search for neuronal mechanisms coupling type 2 diabetes to obesity. J Clin Invest. 2001;108:963–4.
Rodriguez-Diaz R, Caicedo A. Novel approaches to studying the role of innervation in the biology of pancreatic islets. Endocrinol Metab Clin North Am. 2013;42(1):39–56.
Rogers RC, McTigue DM, Hermann GE. Vagal control of digestion: modulation by central neural and peripheral endocrine factors. Neurosci Biobehav Rev. 1996;20(1):57–66.
Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305.
Wuchert F, Ott D, Rafalzik S, Roth J, Gerstberger R. Tumor necrosis factor-[alpha], interleukin-1[beta] and nitric oxide induce calcium transients in distinct populations of cells cultured from the rat area postrema. J Neuroimmunol. 2009;206(1–2):44–51.
Gray TS, O’Donohue TL, Magnuson DJ. Neuropeptide Y innervation of amygdaloid and hypothalamic neurons that project to the dorsal vagal complex in rat. Peptides. 1986;7(2):341–9.
Hou Z, Miao Y, Gao L, Pan H, Zhu S. Ghrelin-containing neuron in cerebral cortex and hypothalamus linked with the DVC of brainstem in rat. Regul Pept. 2006;134(2–3):126–31.
Wu X, Gao J, Yan J, Owyang C, Li Y. Hypothalamus–brain stem circuitry responsible for vagal efferent signaling to the pancreas evoked by hypoglycemia in rat. J Neurophysiol. 2004;91(4):1734–47.
Nasrallah H. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008;13(1):27–35.
Asenjo Lobos C, Komossa K, Rummel-Kluge C, Hunger H, Schmid F, Schwarz S et al. Clozapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010(11):CD006633.
Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(7):1081–90.
Correll CU. From receptor pharmacology to improved outcomes: individualising the selection, dosing, and switching of antipsychotics. Eur Psychiatry. 2010;25(Supplement 2):S12–21.
Scarr E, Dean B. Role of the cholinergic system in the pathology and treatment of schizophrenia. Expert Rev Neurother. 2009;9(1):73–86.
Caulfield MP, Birdsall NJM. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50(2):279–90.
Deng C, Huang X-F. Decreased density of muscarinic receptors in the superior temporal gyrus in schizophrenia. J Neurosci Res. 2005;81(6):883–90.
Dean B, Gray L, Keriakous D, Scarr E. A comparison of M1 and M4 muscarinic receptors in the thalamus from control subjects and subjects with schizophrenia. Thalamus Relat Syst. 2004;2(4):287–95.
Scarr E, Cowie TF, Kanellakis S, Sundram S, Pantelis C, Dean B. Decreased cortical muscarinic receptors define a subgroup of subjects with schizophrenia. Mol Psychiatry. 2008;14(11):1017–23.
Scarr E, Sundram S, Keriakous D, Dean B. Altered hippocampal muscarinic M4, but Not M1, receptor expression from subjects with schizophrenia. Biol Psychiatry. 2007;61(10):1161–70.
Raedler TJ, Bymaster FP, Tandon R, Copolov D, Dean B. Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry. 2006;12(3):232–46.
Deng C, Weston-Green KL, Han M, Huang X-F. Olanzapine treatment decreases the density of muscarinic M2 receptors in the dorsal vagal complex of rats. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(4):915–20.
Scarr E, Keriakous D, Crossland N, Dean B. No change in cortical muscarinic M2, M3 receptors or [35S]GTP[gamma]S binding in schizophrenia. Life Sci. 2006;78(11):1231–7.
Steward LJ, Kennedy MD, Morris BJ, Pratt JA. Chronic phencyclidine (PCP)-induced modulation of muscarinic receptor mRNAs in rat brain : Impact of antipsychotic drug treatment. Neuropharmacology. 2012;62(3):1554–63.
Bymaster F, Felder C. Role of the cholinergic muscarinic system in bipolar disorder and related mechanism of action of antipsychotic agents. Mol Psychiatry. 2002;7(Suppl 1):S57–63.
Jindal R, Keshavan M. Critical role of M3 muscarinic receptor in insulin secretion: implications for psychopharmacology. J Clin Psychopharmacol. 2006;26(5):449–50.
O’Neill MF. Unusual suspect for antipsychotic-induced diabetes. Drug Discov Today. 2005;10(20):1338.
Reynolds GP, Kirk SL. Metabolic side effects of antipsychotic drug treatment - pharmacological mechanisms. Pharmacol Ther. 2010;125(1):169–79.
Starrenburg FCJ, Bogers JPAM. How can antipsychotics cause diabetes mellitus? Insights based on receptor-binding profiles, humoral factors and transporter proteins. Eur Psychiatry. 2009;24(3):164–70.
Silvestre J, Prous J. Research on adverse drug events. I. Muscarinic M3 receptor binding affinity could predict the risk of antipsychotics to induce type 2 diabetes. Methods Find Exp Clin Pharmacol. 2005;27(5):289–304.
Matsui-Sakata A, Ohtani H, Sawada Y. Receptor occupancy-based analysis of the contributions of various receptors to antipsychotics-induced weight gain and diabetes mellitus. Drug Metab Pharmacokinet. 2005;20(5):368–78.
Erle G, Basso M, Federspil G, Sicolo N, Scandellari C. Effect of chlorpromazine on blood glucose and plasma insulin in man. Eur J Clin Pharmacol. 1977;11(1):15–8.
Bhattacharyya J, Das KP. Aggregation of insulin by chlorpromazine. Biochem Pharmacol. 2001;62(9):1293–7.
Ammon HPT, Orci L, Steinke J. Effect of chlorpromazine (CPZ) on insulin release in vivo and in vitro in the rat. J Pharmacol Exp Ther. 1973;187(2):423–9.
Park S, Hong SM, Lee JE, Sung SR. Chlorpromazine exacerbates hepatic insulin sensitivity via attenuating insulin and leptin signaling pathway, while exercise partially reverses the adverse effects. Life Sci. 2007;80(26):2428–35.
Levey AI, Edmunds SM, Heilman CJ, Desmond TJ, Frey KA. Localization of muscarinic M3 receptor protein and M3 receptor binding in rat brain. Neuroscience. 1994;63(1):207–21.
Zubieta JK, Frey KA. Autoradiographic mapping of M3 muscarinic receptors in the rat brain. J Pharmacol Exp Ther. 1993;264(1):415–22.
Gautam D, Jeon J, Starost MF, Han S-J, Hamdan FF, Cui Y, et al. Neuronal M3 muscarinic acetylcholine receptors are essential for somatotroph proliferation and normal somatic growth. Proc Natl Acad Sci. 2009;106(15):6398–403.
Balakrishnan S, Mathew J, Antony S, Paulose CS. Muscarinic M1, M3 receptors function in the brainstem of streptozotocin induced diabetic rats: their role in insulin secretion from the pancreatic islets as a function of age. Eur J Pharmacol. 2009;608(1–3):14–22.
Renuka TR, Ani DV, Paulose CS. Alterations in the muscarinic M1 and M3 receptor gene expression in the brain stem during pancreatic regeneration and insulin secretion in weanling rats. Life Sci. 2004;75(19):2269–80.
Antony S, Kumar TP, Mathew J, Anju TR, Paulose CS. Hypoglycemia induced changes in cholinergic receptor expression in the cerebellum of diabetic rats. J Biomed Sci. 2010;17(1):7.
Martins PJF, Haas M, Obici S. Central nervous system delivery of the antipsychotic olanzapine induces hepatic insulin resistance. Diabetes. 2010;59(10):2418–25.
Weston-Green K, Huang X-F, Lian J, Deng C. Effects of olanzapine on muscarinic M3 receptor binding density in the brain relates to weight gain, plasma insulin and metabolic hormone levels. Eur Neuropsychopharmacol. 2012;22(5):364–73.
Begg DP, Woods SC. Interactions between the central nervous system and pancreatic islet secretions: a historical perspective. Adv Physiol Educ. 2013;37(1):53–60.
Buijs R, Chun S, Niijima A, Romijn H, Nagai K. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J Comp Neurol. 2001;431(4):405–23.
Ruiz de Azua I, Gautam D, Guettier J-M, Wess J. Novel insights into the function of [beta]-cell M3 muscarinic acetylcholine receptors: therapeutic implications. Trends Endocrinol Metabol. 2011;22(2):74–80.
Plamboeck A, Veedfald S, Deacon CF, Hartmann B, Wettergren A, Svendsen LB, et al. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am J Physiol Gastrointest Liver Physiol. 2013;304(12):G1117–27.
Dockray GJ. The versatility of the vagus. Physiol Behav. 2009;97(5):531–6.
Berthoud H-R. The vagus nerve, food intake and obesity. Regul Pept. 2008;149(1–3):15–25.
Ochi Y, Horie S, Maruyama T, Watanabe K, Yano S. Necessity of intracellular cyclic AMP in inducing gastric acid secretion via muscarinic M3 and cholecystokinin2 receptors on parietal cells in isolated mouse stomach. Life Sci. 2005;77(16):2040–50.
Gautam D, de Azua IR, Li JH, Guettier J-M, Heard T, Cui Y, et al. Beneficial metabolic effects caused by persistent activation of {beta}-cell M3 muscarinic acetylcholine receptors in transgenic mice. Endocrinology. 2010;151(11):5185–94.
Gautam D, Gavrilova O, Jeon J, Pack S, Jou W, Cui Y, et al. Beneficial metabolic effects of M3 muscarinic acetylcholine receptor deficiency. Cell Metab. 2006;4(5):363–75.
Gautam D, Han S-J, Hamdan FF, Jeon J, Li B, Li JH, et al. A critical role for [beta] cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006;3(6):449–61.
Gromada J, Hughes TE. Ringing the dinner bell for insulin: muscarinic M3 receptor activity in the control of pancreatic [beta] cell function. Cell Metab. 2006;3(6):390–2.
Zawalich WS, Zawalich KC, Tesz GJ, Taketo MM, Sterpka J, Philbrick W, et al. Effects of muscarinic receptor type 3 knockout on mouse islet secretory responses. Biochem Biophys Res Commun. 2004;315(4):872–6.
Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, et al. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410(6825):207–12.
Ruiz de Azua I, Gautam D, Jain S, Guettier J-M, Wess Jr. Critical metabolic roles of β-cell M3 muscarinic acetylcholine receptors. Life Sci. 2012;91(21–22):986–91.
Ahren B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8(5):369–85.
Kong KC, Butcher AJ, McWilliams P, Jones D, Wess Jr, Hamdan FF et al. M3-muscarinic receptor promotes insulin release via receptor phosphorylation/arrestin-dependent activation of protein kinase D1. Proc Natl Acad Sci USA. 2010;107(49):21181–6.
Duttaroy A, Zimliki CL, Gautam D, Cui Y, Mears D, Wess J. Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in M3 muscarinic acetylcholine receptor-deficient mice. Diabetes. 2004;53(7):1714–20.
Li JH, Gautam D, Han SJ, Guettier JM, Cui Y, Lu H, et al. Hepatic muscarinic acetylcholine receptors are not critically involved in maintaining glucose homeostasis in mice. Diabetes. 2009;58(12):2776–87.
Boschero AC, Szpak-Glasman M, Carneiro EM, Bordin S, Paul I, Rojas E, et al. Oxotremorine-m potentiation of glucose-induced insulin release from rat islets involves M3 muscarinic receptors. Am J Physiol Endocrinol Metab. 1995;268(2):E336–42.
Collins D, Smith DA, Messer WS Jr. Regional binding of 4-diphenylacetoxy-N-methylpiperidine methobromide (4-DAMP) to muscarinic receptors in rat brain and comparative analysis of minimum energy conformations. Neurochem Int. 1993;22(3):237–47.
Hahn M, Chintoh A, Giacca A, Xu L, Lam L, Mann S, et al. Atypical antipsychotics and effects of muscarinic, serotonergic, dopaminergic and histaminergic receptor binding on insulin secretion in vivo: an animal model. Schizophr Res. 2011;131:90–5.
Yamada S, Maruyama S, Takagi Y, Uchida S, Oki T. In vivo demonstration of M3 muscarinic receptor subtype selectivity of darifenacin in mice. Life Sci. 2006;80(2):127–32.
De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2012;8(2):114–26.
Guenette M, Hahn M, Cohn T, Teo C, Remington G. Atypical antipsychotics and diabetic ketoacidosis: a review. Psychopharmacology. 2013;226(1):1–12.
Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, et al. H1-Histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28(3):519–26.
Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors: focus on newer generation compounds. Life Sci. 2000;68(1):29–39.
Acknowledgments
This article was supported by a project grant (APP1044624) from the National Health and Medical Research Council (NHMRC), Australia, and by the Schizophrenia Research Institute, Australia, utilising infrastructure funding from NSW Health. The funding bodies did not play a role in the design, interpretation, writing or submission of this paper. Dr Weston-Green, Professor Huang and Associate Professor Deng declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weston-Green, K., Huang, XF. & Deng, C. Second Generation Antipsychotic-Induced Type 2 Diabetes: A Role for the Muscarinic M3 Receptor. CNS Drugs 27, 1069–1080 (2013). https://doi.org/10.1007/s40263-013-0115-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-013-0115-5