Abstract
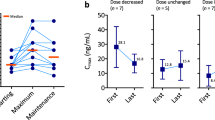
Cabozantinib inhibits receptor tyrosine kinases involved in tumor angiogenesis and metastasis. The capsule formulation (Cometriq®) is approved for the treatment of progressive metastatic medullary thyroid cancer at a 140-mg free base equivalent dose. The tablet formulation (Cabometyx™, 60-mg free base equivalent dose) is approved for the treatment of renal cell carcinoma following anti-angiogenic therapy. Cabozantinib displays a long terminal plasma half-life (~120 h) and accumulates ~fivefold by day 15 following daily dosing based on area under the plasma concentration-time curve (AUC). Four identified inactive metabolites constitute >65 % of total cabozantinib-related AUC following a single 140-mg free base equivalent dose. Cabozantinib AUC was increased by 63–81 % or 7–30 % in subjects with mild/moderate hepatic or renal impairment, respectively; by 34–38 % with concomitant cytochrome P450 3A4 inhibitor ketoconazole; and by 57 % following a high-fat meal. Cabozantinib AUC was decreased by 76–77 % with concomitant cytochrome P450 3A4 inducer rifampin, and was unaffected following administration of proton pump inhibitor esomeprazole. Cabozantinib is a potent in vitro inhibitor of P-glycoprotein, and multidrug and toxin extrusion transporter 1 and 2-K, and is a substrate for multidrug resistance protein 2. No clinically significant covariates affecting cabozantinib pharmacokinetics were identified in a population pharmacokinetic analysis. Patients with medullary thyroid cancer with low model-predicted apparent clearance were more likely to dose hold/reduce cabozantinib early, and had a lower average dose through day 85. However, longitudinal tumor modeling suggests that cabozantinib dose reductions from 140 to 60 mg/day did not markedly reduce tumor growth inhibition in medullary thyroid cancer patients.





Similar content being viewed by others
References
Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298–308.
Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417–27.
Trusolino L, Bertotti A, Comoglio PM. MET signaling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11(12):834–48.
Birchmeier C, Birchmeier W, Gherardi E, et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4(12):915–25.
Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008;7(6):504–16.
Nickerson ML, Jaeger E, Shi Y, et al. Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors. Clin Cancer Res. 2008;14(15):4726–34.
Rini BI. Metastatic renal cell carcinoma: many treatment options, one patient. J Clin Oncol. 2009;27(19):3225–34.
Giubellino A, Linehan WM, Bottaro DP. Targeting the Met signaling pathway in renal cancer. Expert Rev Anticancer Ther. 2009;9(6):785–93.
Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary cancer. J Clin Oncol. 2013;31(29):3639–46.
Cometriq™ (cabozantinib) capsules. US prescribing information. Exelixis, Inc. November 2012.
Choueiri TK, Pal SK, McDermott DF, et al. A phase 1 study of cabozantinib (XL184) in patients with renal cell carcinoma. Ann Oncol. 2014;25(8):1603–8.
Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1814–23.
Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomized, open-label phase 3 trial. Lancet Oncol. 2016;17(7):917–27.
Cabometyx™ (cabozantinib) tablets. US prescribing information. Exelixis, Inc. April 2016.
Cabometyx™ (cabozantinib) tablets. Summary of product characteristics. Ipsen Pharma. September 2016.
ClinicalTrials.gov. Study of cabozantinib (XL184) vs placebo in subjects with hepatocellular carcinoma who have received prior sorafenib. ClinicalTrials.gov Identifier NCT01908426. Available from: https://clinicaltrials.gov/ct2/show/NCT01908426?term=cabozantinib&rank=10. Accessed 26 Sep 2016.
Kurzrock R, Sherman SI, Ball DW, et al. Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29:2660–6.
Nguyen L, Holland J, Miles D, et al. Pharmacokinetic (PK) drug interaction studies of cabozantinib: effect of CYP3A4 inducer rifampin and inhibitor ketoconazole on cabozantinib plasma PK, and effect of cabozantinib on CYP2C8 probe substrate rosiglitazone plasma PK. J Clin Pharmacol. 2015;55(9):1012–23.
Nguyen L, Benrimoh N, Xie Y, Lacy S. Pharmacokinetics of cabozantinib tablet and capsule formulations in healthy adult subjects. Anticancer Drugs. 2016;27(7):669–78.
Nguyen L, Holland J, Ramies D, et al. Effect of renal and hepatic impairment on the pharmacokinetics of cabozantinib. J Clin Pharmacol. 2016;56(9):1130–40.
Nguyen L, Holland J, Mamelock R, et al. Evaluation of the effect of food and gastric pH on the single-dose plasma pharmacokinetics of cabozantinib in healthy adult subjects. J Clin Pharmacol. 2015;55:1293–302.
Lacy S, Hsu B, Miles D, et al. Metabolism and disposition of cabozantinib in healthy male volunteers and pharmacologic characterization of its major metabolites. Drug Metab Dispos. 2015;43:1190–207.
Miles D, Jumbe S, Lacy S, Nguyen L. Population pharmacokinetic model of cabozantinib in patients with medullary thyroid carcinoma and its application to an exposure-response analysis. Clin Pharmacokinet. 2016;55(1):93–105.
Miles DR, Wada DR, Jumbe NL, et al. Population pharmacokinetic/pharmacodynamic modeling of tumor growth kinetics in medullary thyroid cancer patients receiving cabozantinib. Anticancer Drugs. 2016;27(4):328–41.
Center for Drug Evaluation and Research. Clinical pharmacology and biopharmaceutics review[s] for cabozantinib (Cometriq), 2012a. Available from: www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203756Orig1s000ClinPharmR.pdf. Accessed 26 Sept 2016.
Bentzien F, Zuzow M, Heald N, et al. In vitro and in vivo activity of cabozantinib (XL184), an inhibitor of RET, MET, and VEGFR2, in a model of medullary thyroid cancer. Thyroid. 2013;23(12):1569–77.
Zhou L, Liu X-D, Sun M, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. 2016;35(21):2687–97.
Xiang Q, Chen W, Ren M, et al. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by dual blockade of VEGFR2 and MET. Clin Cancer Res. 2014;20(11):2959–70.
Sameni M, Tovar EA, Essenburg CJ, et al. Cabozantinib (XL184) inhibits growth and invasion of preclinical TNBC models. Clin Cancer Res. 2016;22(4):923–34.
Zhang L, Scorsone K, Woodfield SE, Page PE. Sensitivity of neuroblastoma to the novel kinase inhibitor cabozantinib is mediated by ERK inhibition. Cancer Chemother Pharmacol. 2015;76(5):977–87.
Song EK, Tai WM, Messersmith WA, et al. Potent antitumor activity of cabozantinib, a c-MET and VEGFR2 inhibitor, in colorectal cancer patient-derived tumor explant model. Int J Cancer. 2015;136(8):1967–75.
Cohen NA, Zeng S, Seifert AM, et al. Pharmacological inhibition of KIT activates MET signaling in gastrointestinal stromal tumors. Cancer Res. 2015;75(10):2061–70.
Sennino B, Ishiguro-Oonuma T, Wei Y, et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov. 2012;2(3):270–87.
Dai J, Zhang H, Karatsinides A, et al. Cabozantinib inhibits prostate cancer growth and prevents tumor-induced bone lesions. Clin Cancer Res. 2014;20(3):617–30.
European Medicines Agency. European Public Assessment Report (EPAR) for Cometriq, 2013. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002640/WC500163705.pdf. Accessed 26 Sep 2016.
European Medicines Agency. Guideline on the investigation of bioequivalence. CPMP/EWP/QWP/1401/98 Rev. 1/Corr. London: European Medicines Agency; January 2010.
US Food and Drug Administration. Guidance for Industry. Bioavailability and bioequivalence studies for orally administered drug products: general considerations; revision 1. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration; 2003.
US Food and Drug Administration. Guidance for industry. Bioavailability and bioequivalence studies submitted in NDAs and INDs: general considerations. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration; 2014.
Foxx-Lupo WT, Sing S, Alwan L, Tykodi SS. A drug interaction between cabozantinib and warfarin in a patient with renal cell carcinoma. Clin Genitourin Cancer. 2016;14(1):119–21.
European Medicines Agency. Guideline on the investigation of drug interactions. London: European Medicines Agency; 2012.
US Food and Drug Administration. Guidance for industry. Drug interactions studies: study design, data analysis, implications for dosing, and labeling recommendations. Rockville, MD: US Food and Drug Administration; 2012.
Xiang QF, Zhang DM, Wang JN, et al. Cabozantinib reverses multidrug resistance of human hepatoma HepG2/adr cells by modulating the function of P-glycoprotein. Liver Int. 2015;35(3):110–23.
Acknowledgments
The authors wish to respectfully thank the medical professionals, patients and healthy volunteers that particpated in the clinical trials of cabozantinib reported in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The studies described in this manuscript were supported by Exelixis, Inc.
Conflict of interest
Steven A. Lacy is a stockholder and current employee of Exelixis, Inc. Dale R. Miles and Linh T. Nguyen were employees of Exelixis, Inc. when this work was performed. The authors contributed significantly to the design, conduct, analyses, and interpretation of the data, and were involved in the preparation, review, and approval of this manuscript.
Ethical approval
The clinical studies were conducted in accordance with the World Medical Association Declaration of Helsinki, the International Conference on Harmonisation Tripartite Guideline for Good Clinical Practice, and all applicable local regulations. Study protocols and informed consent documents were reviewed and approved by the institutional review board of participating institutions, and informed consent was obtained from all participants before any study-specified procedures were undertaken. All animal experiments were performed in facilities accredited by the Association for Assessment and Accreditation of Laboratory Care according to protocols approved by Institutional Animal Care and Use Committees.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lacy, S.A., Miles, D.R. & Nguyen, L.T. Clinical Pharmacokinetics and Pharmacodynamics of Cabozantinib. Clin Pharmacokinet 56, 477–491 (2017). https://doi.org/10.1007/s40262-016-0461-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0461-9