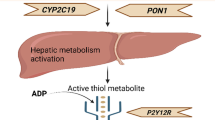
Abstract
Background and Objective
The search for potential gene loci that affect the pharmacodynamics and pharmacokinetics of ticagrelor is a matter of broad clinical interest. The objective of this study was to investigate the effect of genetic polymorphisms on the pharmacokinetics and pharmacodynamics of ticagrelor in healthy Chinese subjects.
Methods
This is a multi-center study in China, including three hospitals from Beijing, Nanchang, and Changsha. Healthy Chinese subjects aged 18–45 years with unknown genotypes were included. All subjects received a single oral dose of 90 mg of ticagrelor. Platelet aggregation and the area under the concentration–time curve for ticagrelor and its major active metabolite in plasma samples were assessed. Genome-wide association studies and candidate gene association analysis related to ticagrelor were performed.
Results
One hundred and seventy-five native Chinese subjects were enrolled and completed the study. According to the p value, the threshold of ticagrelor population was 6.57 × 10−7 (0.05/76106), one single-nucleotide polymorphism chr6:17616513 of gene NUP153 (p = 2.03 × 10−7) related to the area under the concentration–time curve for plasma concentration at time zero versus the last measurable timepoint, and one single nucleotide polymorphism rs17204533 of gene SVEP1 (p = 3.96 × 10−7) related to P2Y12 reaction unit12h of ticagrelor was identified. In addition, L1TD1, CETP, CLEC2A, CHSY1, PDZRN3, CTU2, PIEZO1, APOBEC1, SEMA6A, KAZN, and FASN polymorphisms might influence the pharmacokinetics of ticagrelor, while PARP10, TRIB1, CYP2C19, and UGT2B7 might affected its pharmacodynamics.
Conclusions
Genetic variation affects the pharmacokinetics and pharmacodynamics of ticagrelor in healthy individuals. The detection of NUP153, SVEP1 gene variation will be helpful for pharmacodynamic prediction and evaluation, and the regulation of these genes may be the target of new drug development. Further studies are required to confirm the results and explore whether these single-nucleotide polymorphisms are associated only with platelet activity or also with cardiovascular events and all-cause mortality.
Clinical Trial Registration
NCT03161002.



Similar content being viewed by others
References
Alexopoulos D, Xanthopoulou I, Siapika A, et al. Evolving pattern of on-prasugrel and on-ticagrelor platelet reactivity over time in ST elevation myocardial infarction patients. Int J Cardiol. 2013;168:629–30.
Storey RF, Husted S, Harrington RA, Heptinstall S, Wilcox RG, Peters G, et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol. 2007;50:1852–6.
Teng R, Oliver S, Hayes MA, Butler K. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos. 2010;38:1514–21.
Teng R, Butler K. Pharmacokinetics, pharmacodynamics, tolerability and safety of single ascending doses of ticagrelor, a reversibly binding oral P2Y12 receptor antagonist, in healthy subjects. Eur J Clin Pharmacol. 2010;66:487–96.
Cassese S, Ndrepepa G, Byrne RA, Laugwitz KL, Schunkert H, Fusaro M, et al. Ticagrelor-based antiplatelet regimens in patients with atherosclerotic artery disease: a meta-analysis of randomized clinical trials. Am Heart J. 2020;219:109–16.
Johnston SC, Amarenco P, Denison H, Evans SR, Himmelmann A, James S, THALES Investigators, et al. Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. N Engl J Med. 2020;383(3):207–17.
Angiolillo DJ, Rollini F, Storey RF, et al. International expert consensus on switching platelet P2Y12 receptor-inhibiting therapies. Circulation. 2017;136:1955–75.
Zettler ME, Peterson ED, McCoy LA, et al. Switching of adenosine diphosphate receptor inhibitor after hospital discharge among myocar dial infarction patients: insights from the Treatment With Adenosine Diphosphate Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events After Acute Coronary Syndrome (TRANSLATE-ACS) observational study. Am Heart J. 2017;183:62–8.
Sibbing D, Aradi D, Alexopoulos D, Ten Berg J, Bhatt DL, Bonello L, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12(16):1521–37.
Infeld M, Friede KA, San TR, Knickerbocker HJ, Ginsburg GS, Ortel TL, et al. Platelet reactivity in response to aspirin and ticagrelor in African–Americans and European–Americans. J Thromb Thrombolysis. 2021;51(2):249–59.
Liu S, Shi X, Tian X, Zhang X, Sun Z, Miao L. Effect of CYP3A4∗1G and CYP3A5∗3 polymorphisms on pharmacokinetics and pharmacodynamics of ticagrelor in healthy Chinese subjects. Front Pharmacol. 2017;31(8):176.
Storey RF, Thornton SM, Lawrance R, Husted S, Wickens M, Emanuelsson H, et al. Ticagrelor yields consistent dose-dependent inhibition of ADP-induced platelet aggregation in patients with atherosclerotic disease regardless of genotypic variations in P2RY12, P2RY1, and ITGB3. Platelets. 2009;20:341–8.
Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, PLATO Investigators, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–8.
Tantry US, Bliden KP, Wei C, Storey RF, Armstrong M, Butler K, et al. First analysis of the relation between CYP2C19 genotype and pharmacodynamics in patients treated with ticagrelor versus clopidogrel: the ONSET/OFFSET and RESPOND genotype studies. Circ Cardiovasc Genet. 2010;3:566–666.
Varenhorst C, Eriksson N, Johansson A, Barratt BJ, Hagstrom E, Akerblom A, PLATO Investigators, et al. Effect of genetic variations on ticagrelor plasma levels and clinical outcomes. Eur Heart J. 2015;36:1901–12.
Li M, Hu Y, Li H, Wen Z, Hu X, Zhang D, et al. No effect of SLCO1B1 and CYP3A4/5 polymorphisms on the pharmacokinetics and pharmacodynamics of ticagrelor in healthy Chinese male subjects. Biol Pharm Bull. 2017;40(1):88–96.
Patnala R, Clements J, Batra J. Candidate gene association studies: a comprehensive guide to useful in silico tools. BMC Genet. 2013;9(14):39.
David S. A current guide to candidate gene association studies. Trends Genet. 2021;37(12):1056–9.
Sillén H, Cook M, Davis P. Determination of ticagrelor and two metabolites in plasma samples by liquid chromatography and mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2299–306.
Zhou L, Panté N. The nucleoporin Nup153 maintains nuclear envelope architecture and is required for cell migration in tumor cells. FEBS Lett. 2010;584(14):3013–20.
Leone L, Colussi C, Gironi K, Longo V, Fusco S, Li Puma DD, et al. Altered Nup153 expression impairs the function of cultured hippocampal neural stem cells isolated from a mouse model of Alzheimer’s disease. Mol Neurobiol. 2019;56(8):5934–49.
Choudhary RK, Capuco AV. Expression of NR5A2, NUP153, HNF4A, USP15 and FNDC3B is consistent with their use as novel biomarkers for bovine mammary stem/progenitor cells. J Mol Histol. 2021;52(2):289–300.
Re A, Colussi C, Nanni S, Aiello A, Bacci L, Grassi C, et al. Nucleoporin 153 regulates estrogen dependent nuclear translocation of endothelial nitric oxide synthase and estrogen receptor beta in prostate cancer. Oncotarget. 2018;9(46):27985–97.
Kodiha M, Tran D, Qian C, Morogan A, Presley JF, Brown CM, et al. Oxidative stress mislocalizes and retains transport factor importin-alpha and nucleoporins Nup153 and Nup88 in nuclei where they generate high molecular mass complexes. Biochim Biophys Acta. 2008;1783(3):405–18.
Gasomediators OB. (NO, CO, and H2S) and their role in hemostasis and thrombosis. Clin Chim Acta. 2015;20(445):115–21.
Sato-Nishiuchi R, Nakano I, Ozawa A, Sato Y, Takeichi M, Kiyozumi D, et al. Polydom/SVEP1 is a ligand for integrin α9β1. J Biol Chem. 2012;287:25615–30.
Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia Investigators, Stitziel NO, Stirrups KE, et al. Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N Engl J Med. 2016;374(12):1134–44.
Winkler MJ, Müller P, Sharifi AM, Wobst J, Winter H, Mokry M, et al. Functional investigation of the coronary artery disease gene SVEP1. Basic Res Cardiol. 2020;115(6):67.
Xi Z, Zhou Y, Zhao Y, Liu X, Liang J, Chai M, et al. Ticagrelor versus clopidogrel in patients with two CYP2C19 loss-of-function alleles undergoing percutaneous coronary intervention. Cardiovasc Drugs Ther. 2020;34(2):179–88.
Pereira NL, Farkouh ME, So D, Lennon R, Geller N, Mathew V, et al. Effect of genotype-guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: the TAILOR-PCI randomized clinical trial. JAMA. 2020;324(8):761–71.
Varenhorst C, Eriksson N, Johansson Å, Barratt BJ, Hagström E, Åkerblom A, PLATO Investigators, et al. Effect of genetic variations on ticagrelor plasma levels and clinical outcomes. Eur Heart J. 2015;36(29):1901–12.
Iwanicka J, Iwanicki T, Niemiec P, Balcerzyk A, Krauze J, Górczyńska-Kosiorz S, et al. Relationship between CETP gene polymorphisms with coronary artery disease in Polish population. Mol Biol Rep. 2018;45(6):1929–35.
Vrkić Kirhmajer M, Macolić Šarinić V, Šimičević L, Ladić I, Putarek K, Banfić L, et al. Rosuvastatin-induced rhabdomyolysis: possible role of ticagrelor and patients’ pharmacogenetic profile. Basic Clin Pharmacol Toxicol. 2018;123(4):509–18.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by Grants from the National Key R&D Program of China (2016YFC0904900), National Natural Science Foundation of China (81872940, 81973395 and 82073935), and Beijing Municipal Commission of Science and Technology of China Pharmaceutical Innovation Cultivation and Industry Support Platform Capacity Construction Project (Z191100007619038).
Conflict of interest
We declare no competing interests.
Ethics approval
The protocol was approved by an independent ethics committee and the Institutional Review Board of Peking University First Hospital and all participating research sub-central hospitals. The trial registration number is NCT03161002.
Consent to participate
All subjects were enrolled in this study after signing the informed consent.
Consent for publication
Not applicable.
Availability of data and material
All data related to the study have been shown in the article, and other relevant data can be communicated with the author.
Code availability
Not applicable.
Authors′ Contribution
(1) Conception and design: Yimin Cui, Guoping Yang, Ninghong Guo, Jie Huang, Jie Jiang and Jian Li; (2) Provision of study materials or patients: Qian Xiang, Zhiyan Liu, Guangyan Mu, Qiufen Xie, Hanxu Zhang and Shuang Zhou; (3) Collection and assembly of data: Zhiyan Liu and Zining Wang; (4) Data analysis and interpretation: Zhiyan Liu, Qiufen Xie, Hanxu Zhang and Qian Xiang; (5) Manuscript writing and Final approval of manuscript: All authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiang, Q., Liu, Z., Mu, G. et al. Effect of Genetic Polymorphism Including NUP153 and SVEP1 on the Pharmacokinetics and Pharmacodynamics of Ticagrelor in Healthy Chinese Subjects. Clin Drug Investig 42, 447–458 (2022). https://doi.org/10.1007/s40261-022-01154-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-022-01154-6