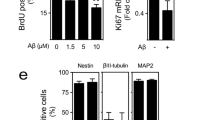
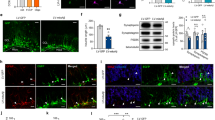
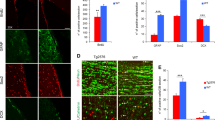
Abstract
Impairment of adult hippocampal neurogenesis is an early event in Alzheimer’s disease (AD), playing a crucial role in cognitive dysfunction associated with this pathology. However, the mechanisms underlying defective neurogenesis in AD are still unclear. Recently, the nucleoporin Nup153 has been described as a new epigenetic determinant of adult neural stem cell (NSC) maintenance and fate. Here we investigated whether Nup153 dysfunction could affect the plasticity of NSCs in AD. Nup153 expression was strongly reduced in AD-NSCs, as well as its interaction with the transcription factor Sox2, a master regulator of NSC stemness and their neuronal differentiation. Similar Nup153 reduction was also observed in WT-NSCs treated with amyloid-β (Aβ) or stimulated with a nitric oxide donor. Accordingly, AD-NSCs treated with either a γ-secretase inhibitor or antioxidant compounds showed higher Nup153 levels suggesting that both nitrosative stress and Aβ accumulation affect Nup153 expression. Of note, restoration of Nup153 levels in AD-NSCs promoted their proliferation, as assessed by BrdU incorporation, neurosphere assay, and stemness gene expression analysis. Nup153 overexpression also recovered AD-NSC response to differentiation, increasing the expression of pro-neuronal genes, the percentage of cells positive for neuronal markers, and the acquisition of a more mature neuronal phenotype. Electrophysiological recordings revealed that neurons differentiated from Nup153-transfected AD-NSCs displayed higher Na+ current density, comparable to those deriving from WT-NSCs. Our data uncover a novel role for Nup153 in NSCs from animal model of AD and point to Nup153 as potential target to restore physiological NSC behavior and fate in neurodegenerative diseases.





Similar content being viewed by others
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article and in the additional files.
Abbreviations
- AD :
-
Alzheimer’s disease
- Nup153 :
-
nucleoporin153
- NSCs :
-
neural stem cells
- NO :
-
nitric oxide
- NAC :
-
N-acetylcysteine
- AA :
-
ascorbic acid
- LNAME :
-
L-NG-nitro-arginine methyl ester
- GSI :
-
γ-secretase inhibitor
- MFI :
-
mean fluorescence intensity
- TLX/NR2E1 :
-
nuclear receptor subfamily 2 group E member 1
References
Braun SM, Jessberger S (2014) Adult neurogenesis: mechanisms and functional significance. Development 141(10):1983–1986. https://doi.org/10.1242/dev.104596
Toda T, Gage FH (2017) Review: adult neurogenesis contributes to hippocampal plasticity. Cell Tissue Res 373:693–709. https://doi.org/10.1007/s00441-017-2735-4
Caselli RJ, Beach TG, Yaari R, Reiman EM (2006) Alzheimer’s disease a century later. J Clin Psychiatry 67(11):1784–1800
Hamilton LK, Aumont A, Julien C, Vadnais A, Calon F, Fernandes KJ (2010) Widespread deficits in adult neurogenesis precede plaque and tangle formation in the 3xTg mouse model of Alzheimer’s disease. Eur J Neurosci 32(6):905–920. https://doi.org/10.1111/j.1460-9568.2010.07379.x
Lazarov O, Hollands C (2016) Hippocampal neurogenesis: learning to remember. Prog Neurobiol 138-140:1–18. https://doi.org/10.1016/j.pneurobio.2015.12.006
Fitzsimons CP, van Bodegraven E, Schouten M, Lardenoije R, Kompotis K, Kenis G, van den Hurk M, Boks MP et al (2014) Epigenetic regulation of adult neural stem cells: implications for Alzheimer’s disease. Mol Neurodegener 9:25. https://doi.org/10.1186/1750-1326-9-25
Raices M, D'Angelo MA (2017) Nuclear pore complexes and regulation of gene expression. Curr Opin Cell Biol 46:26–32. https://doi.org/10.1016/j.ceb.2016.12.006
Palancade B, Doye V (2008) Sumoylating and desumoylating enzymes at nuclear pores: underpinning their unexpected duties? Trends Cell Biol 18(4):174–183. https://doi.org/10.1016/j.tcb.2008.02.001
Nanni S, Re A, Ripoli C, Gowran A, Nigro P, D'Amario D, Amodeo A, Crea F et al (2016) The nuclear pore protein Nup153 associates with chromatin and regulates cardiac gene expression in dystrophic mdx hearts. Cardiovasc Res 112(2):555–567. https://doi.org/10.1093/cvr/cvw204
Re A, Colussi C, Nanni S, Aiello A, Bacci L, Grassi C, Pontecorvi A, Farsetti A (2018) Nucleoporin 153 regulates estrogen-dependent nuclear translocation of endothelial nitric oxide synthase and estrogen receptor beta in prostate cancer. Oncotarget 9(46):27985–27997. https://doi.org/10.18632/oncotarget.25462
Jacinto FV, Benner C, Hetzer MW (2015) The nucleoporin Nup153 regulates embryonic stem cell pluripotency through gene silencing. Genes Dev 29(12):1224–1238. https://doi.org/10.1101/gad.260919.115
Toda T, Hsu JY, Linker SB, Hu L, Schafer ST, Mertens J, Jacinto FV, Hetzer MW et al (2017) Nup153 interacts with Sox2 to enable bimodal gene regulation and maintenance of neural progenitor cells. Cell Stem Cell 21:618–634.e7. https://doi.org/10.1016/j.stem.2017.08.012
Liu GH, Li M, Qu J, Izpisua Belmonte JC (2012) Gating neural development and aging via nuclear pores. Cell Res 22(8):1212–1214. https://doi.org/10.1038/cr.2012.35
Zhang J, Snyder SH (1995) Nitric oxide in the nervous system. Annu Rev Pharmacol Toxicol 35:213–233. https://doi.org/10.1146/annurev.pa.35.040195.001241
Podda MV, Marcocci ME, Oggiano L, D'Ascenzo M, Tolu E, Palamara AT, Azzena GB, Grassi C (2004) Nitric oxide increases the spontaneous firing rate of rat medial vestibular nucleus neurons in vitro via a cyclic GMP-mediated PKG-independent mechanism. Eur J Neurosci 20(8):2124–2132. https://doi.org/10.1111/j.1460-9568.2004.03674.x
Licht T, Keshet E (2015) The vascular niche in adult neurogenesis. Mech Dev 138(Pt 1):56–62. https://doi.org/10.1016/j.mod.2015.06.001
Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y et al (2006) S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 441(7092):513–517. https://doi.org/10.1038/nature04782
He P, Shen Y (2009) Interruption of beta-catenin signaling reduces neurogenesis in Alzheimer’s disease. J Neurosci 29(20):6545–6557. https://doi.org/10.1523/JNEUROSCI.0421-09.2009
Pajak B, Kania E, Orzechowski A (2016) Killing me softly: connotations to unfolded protein response and oxidative stress in Alzheimer’s disease. Oxidative Med Cell Longev 2016:1805304–1805317. https://doi.org/10.1155/2016/1805304
Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A (2008) S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature 455(7211):411–415. https://doi.org/10.1038/nature07238
Colussi C, Mozzetta C, Gurtner A, Illi B, Rosati J, Straino S, Ragone G, Pescatori M et al (2008) HDAC2 blockade by nitric oxide and histone deacetylase inhibitors reveals a common target in Duchenne muscular dystrophy treatment. Proc Natl Acad Sci U S A 105(49):19183–19187. https://doi.org/10.1073/pnas.0805514105
Re A, Colussi C, Nanni S, Aiello A, Bacci L, Grassi C, Pontecorvi A, Farsetti A (2018) Nucleoporin 153 regulates estrogen dependent nuclear translocation of endothelial nitric oxide synthase and estrogen receptor beta in prostate cancer. Oncotarget 9(46):27985–27997
Kodiha M, Chu A, Matusiewicz N, Stochaj U (2004) Multiple mechanisms promote the inhibition of classical nuclear import upon exposure to severe oxidative stress. Cell Death Differ 11(8):862–874. https://doi.org/10.1038/sj.cdd.4401432
Kodiha M, Tran D, Qian C, Morogan A, Presley JF, Brown CM, Stochaj U (2008) Oxidative stress mislocalizes and retains transport factor importin-alpha and nucleoporins Nup153 and Nup88 in nuclei where they generate high molecular mass complexes. Biochim Biophys Acta 1783(3):405–418. https://doi.org/10.1016/j.bbamcr.2007.10.022
Mastrodonato A, Barbati SA, Leone L, Colussi C, Gironi K, Rinaudo M, Piacentini R, Denny CA et al (2018) Olfactory memory is enhanced in mice exposed to extremely low-frequency electromagnetic fields via Wnt/beta-catenin dependent modulation of subventricular zone neurogenesis. Sci Rep 8(1):262. https://doi.org/10.1038/s41598-017-18676-1
Puzzo D, Piacentini R, Fa M, Gulisano W, Li Puma DD, Staniszewski A, Zhang H, Tropea MR et al (2017) LTP and memory impairment caused by extracellular Abeta and Tau oligomers is APP-dependent. Elife 6. https://doi.org/10.7554/eLife.26991
Nanni S, Aiello A, Re A, Guffanti A, Benvenuti V, Colussi C, Castro-Vega LJ, Felsani A et al (2013) Estrogen-dependent dynamic profile of eNOS-DNA associations in prostate cancer. PLoS One 8(5):e62522. https://doi.org/10.1371/journal.pone.0062522
Nanni S, Benvenuti V, Grasselli A, Priolo C, Aiello A, Mattiussi S, Colussi C, Lirangi V et al (2009) Endothelial NOS, estrogen receptor beta, and HIFs cooperate in the activation of a prognostic transcriptional pattern in aggressive human prostate cancer. J Clin Invest 119(5):1093–1108. https://doi.org/10.1172/JCI35079
Re A, Aiello A, Nanni S, Grasselli A, Benvenuti V, Pantisano V, Strigari L, Colussi C et al (2011) Silencing of GSTP1, a prostate cancer prognostic gene, by the estrogen receptor-beta and endothelial nitric oxide synthase complex. Mol Endocrinol 25(12):2003–2016. https://doi.org/10.1210/me.2011-1024
Spinelli M, Fusco S, Mainardi M, Scala F, Natale F, Lapenta R, Mattera A, Rinaudo M et al (2017) Brain insulin resistance impairs hippocampal synaptic plasticity and memory by increasing GluA1 palmitoylation through FoxO3a. Nat Commun 8(1):2009. https://doi.org/10.1038/s41467-017-02221-9
Aceto G, Re A, Mattera A, Leone L, Colussi C, Rinaudo M, Scala F, Gironi K et al (2018) GSK3beta modulates timing-dependent long-term depression through direct phosphorylation of Kv4.2 channels. Cereb Cortex. https://doi.org/10.1093/cercor/bhy042
Biella G, Di Febo F, Goffredo D, Moiana A, Taglietti V, Conti L, Cattaneo E, Toselli M (2007) Differentiating embryonic stem-derived neural stem cells show a maturation-dependent pattern of voltage-gated sodium current expression and graded action potentials. Neuroscience 149(1):38–52. https://doi.org/10.1016/j.neuroscience.2007.07.021
Haughey NJ, Liu D, Nath A, Borchard AC, Mattson MP (2002) Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer’s disease. NeuroMolecular Med 1(2):125–135. https://doi.org/10.1385/NMM:1:2:125
Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G et al (2010) Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 6(5):445–456. https://doi.org/10.1016/j.stem.2010.03.017
Hagey DW, Muhr J (2014) Sox2 acts in a dose-dependent fashion to regulate proliferation of cortical progenitors. Cell Rep 9(5):1908–1920. https://doi.org/10.1016/j.celrep.2014.11.013
Schmid RS, Maness PF (2008) L1 and NCAM adhesion molecules as signaling coreceptors in neuronal migration and process outgrowth. Curr Opin Neurobiol 18(3):245–250. https://doi.org/10.1016/j.conb.2008.07.015
Islam MM, Zhang CL (2015) TLX: a master regulator for neural stem cell maintenance and neurogenesis. Biochim Biophys Acta 1849(2):210–216. https://doi.org/10.1016/j.bbagrm.2014.06.001
Sun G, Cui Q, Shi Y (2017) Nuclear receptor TLX in development and diseases. Curr Top Dev Biol 125:257–273. https://doi.org/10.1016/bs.ctdb.2016.12.003
Serini S, Calviello G (2016) Reduction of oxidative/nitrosative stress in brain and its involvement in the neuroprotective effect of n-3 PUFA in Alzheimer’s disease. Curr Alzheimer Res 13(2):123–134
Swomley AM, Butterfield DA (2015) Oxidative stress in Alzheimer disease and mild cognitive impairment: evidence from human data provided by redox proteomics. Arch Toxicol 89(10):1669–1680. https://doi.org/10.1007/s00204-015-1556-z
Molokanova E, Akhtar MW, Sanz-Blasco S, Tu S, Pina-Crespo JC, McKercher SR, Lipton SA (2014) Differential effects of synaptic and extrasynaptic NMDA receptors on Abeta-induced nitric oxide production in cerebrocortical neurons. J Neurosci 34(14):5023–5028. https://doi.org/10.1523/JNEUROSCI.2907-13.2014
Tajes M, Eraso-Pichot A, Rubio-Moscardo F, Guivernau B, Ramos-Fernandez E, Bosch-Morato M, Guix FX, Clarimon J et al (2014) Methylglyoxal produced by amyloid-beta peptide-induced nitrotyrosination of triosephosphate isomerase triggers neuronal death in Alzheimer’s disease. J Alzheimers Dis 41(1):273–288. https://doi.org/10.3233/JAD-131685
Dias C, Lourenco CF, Ferreiro E, Barbosa RM, Laranjinha J, Ledo A (2016) Age-dependent changes in the glutamate-nitric oxide pathway in the hippocampus of the triple transgenic model of Alzheimer’s disease: implications for neurometabolic regulation. Neurobiol Aging 46:84–95. https://doi.org/10.1016/j.neurobiolaging.2016.06.012
Lourenco CF, Ledo A, Barbosa RM, Laranjinha J (2017) Neurovascular uncoupling in the triple transgenic model of Alzheimer’s disease: impaired cerebral blood flow response to neuronal-derived nitric oxide signaling. Exp Neurol 291:36–43. https://doi.org/10.1016/j.expneurol.2017.01.013
Shariatpanahi M, Khodagholi F, Ashabi G, Aghazadeh Khasraghi A, Azimi L, Abdollahi M, Ghahremani MH, Ostad SN et al (2015) Ameliorating of memory impairment and apoptosis in amyloid beta-injected rats via inhibition of nitric oxide synthase: possible participation of autophagy. Iran J Pharm Res 14(3):811–824
Diaz A, Rojas K, Espinosa B, Chavez R, Zenteno E, Limon D, Guevara J (2014) Aminoguanidine treatment ameliorates inflammatory responses and memory impairment induced by amyloid-beta 25-35 injection in rats. Neuropeptides 48(3):153–159. https://doi.org/10.1016/j.npep.2014.03.002
Austin SA, Santhanam AV, Hinton DJ, Choi DS, Katusic ZS (2013) Endothelial nitric oxide deficiency promotes Alzheimer’s disease pathology. J Neurochem 127(5):691–700. https://doi.org/10.1111/jnc.12334
Nagai N, Ito Y, Shibata T, Kubo E, Sasaki H (2017) A positive feedback loop between nitric oxide and amyloid beta (1-42) accelerates mitochondrial damage in human lens epithelial cells. Toxicology 381:19–30. https://doi.org/10.1016/j.tox.2017.02.014
Ferreira NR, Ledo A, Laranjinha J, Gerhardt GA, Barbosa RM (2018) Simultaneous measurements of ascorbate and glutamate in vivo in the rat brain using carbon fiber nanocomposite sensors and microbiosensor arrays. Bioelectrochemistry 121:142–150. https://doi.org/10.1016/j.bioelechem.2018.01.009
Acknowledgements
We thank Professor D. Puzzo, from University of Catania, who kindly provided tissues from APP knock-out mice (B6.129S7-Apptm1Dbo/J; 4 months old) that were used as negative control in Western blot experiments.
Author information
Authors and Affiliations
Contributions
Electrophysiology: MD, VL; RT-qPCR: KG, LL, SF; ChIP: SF; NSC experiments and analysis: LL, KG, CC; confocal analysis: CC; WB and IP: CC, DDLP; conceptualization: LL, CC, CG; writing: LL, CC, CG. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Mice were used in agreement with the guidelines of the European Parliament (Directive 2010/63/EU for the protection of laboratory animals) and with the guidelines of the Italian National Institute of Health and were approved by the Institutional Animal Care of Università Cattolica (approval number: 553/2016PR, Rome, Italy).
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplementary Fig. 1
Evaluation of Nup153 levels in GFAP positive radial and non-radial NSCs in the hippocampus from WT and AD mice. a) Representative images showing the merged signals for Nup153, GFAP and DAPI immunostaining in the DG of the hippocampus from WT and AD mice (scale bar 50 μm). b) Representative images showing Nup153, Nup153/GFAP or merged signals with DAPI of DG from WT and AD mice at higher magnification (scale bar 10 μm). c) Mean fluorescence intensity (MFI) for Nup153 levels in GFAP positive cells showing radial or non-radial morphology (ANOVA, Bonferroni test, n=4). Data plotted as mean ± SEM, ***p<0.001. (PDF 10710 kb)
Supplementary Fig. 2
Aβ levels in AD-NSCs. a) Analysis of Aβ levels by dot blot in WT- and AD-NSCs treated with vehicle or GSI (1μM) for 72 h. The lower panel shows the densitometry of Aβ signal normalized to red ponceau staining (ANOVA, Bonferroni test, n=6). b) Representative WB showing the presence of small oligomers (trimers and tetramers are indicated by arrowheads) in hippocampal tissue from APP-KO (negative control) or 3×Tg (positive control) mice and in AD-NSCs compared with synthetic Aβ preparation (200 nM). RP: red ponceau staining. Data plotted as mean ± SEM, ***p<0.001. (PDF 595 kb)
Supplementary Fig. 3
Effect of LNAME treatment on nitro-tyrosine levels in AD-NSCs. a) Analysis of nitro-tyrosine levels by dot blot in AD-NSCs treated with LNAME (LN) for 72h and compared to vehicle treated-AD-NSCs. RP: red ponceau staining. b) Densitometry of nitro-tyrosine signal normalized to red ponceau staining (RP). (Student’s t test, n=4). Data plotted as mean ± SEM, ***p<0.001. (PDF 1189 kb)
Supplementary Fig. 4
Nup153 modulation regulates neurosphere formation in WT-NSCs. a) Confocal analysis showing Nup153 expression in scramble- (sc) or Nup153 silenced-neurospheres (siNup153) and GFP signal in GFP- or GFP-Nup153 transfected neurospheres (scale bar 50 μm). Nuclei were counterstained with DAPI. b) Neurosphere assay in WT-NSCs treated with: scramble- or siNup153 oligos, GFP- or GFP-Nup153 vectors (mean±SEM, ***p<0.001, ANOVA, Bonferroni test, n=4). (PDF 2264 kb)
Supplementary Fig. 5
Evaluation of transfection efficiency in AD-NSCs. a) Representative images showing the level of GFP in neurospheres transfected either with GFP or GFP-Nup153 vectors. Nuclei were counterstained with DAPI, scale bar 50 μm. The graph shows the percentage of GFP+ cells in both conditions. Data plotted as mean ± SEM, n=4). (PDF 2344 kb)
Supplementary Fig. 6
Nup153 overexpression increases histone H3 acetylation level in AD-NSCs. Representative images showing the level of acetylation on lysine 9 and 14 of histone H3 (H3K9-14ac) in neurospheres isolated from AD mice overexpressing Nup153 compared to GFP-controls. Nuclei were counterstained with DAPI, scale bar 50 μm. (PDF 2447 kb)
Supplementary Fig. 7
Nup153 silencing modulates differentiation of WT-NSCs. a) Confocal analysis of Nup153 and DCX expression in proliferating WT-NSCs and after two days of differentiation (D2) (scale bar 20 μm). b) RT-qPCR showing the expression levels of Mef2c, Mash1 and NeuroD1 in scramble or siNup153-treated WT-NSCs. (Student’s t test, n=3). c) Evaluation of β–III tubulin and MAP2 expression at D6 in scramble- or siNup153-treated WT-NSCs. Nuclei were counterstained with DAPI (scale bar 50 μm). d) Neurite length in scramble- or siNup153-treated WT-NSCs at D10 (Student’s t test, n=5) e) Representative traces of voltage-gated Na+ currents evoked by a series of depolarizing steps from -90 to +40 mV in scramble- or siNup153 treated WT-NSCs. Inset: voltage protocols. The right panel shows current-voltage relationship of peak Na+ current densities for NSCs in the above conditions (black circles, scramble; red circles, siNup153; Student’s t test, scramble n=25, siNup n=18). Data plotted as mean ± SEM, *p<0.05, **p<0.01. (PDF 6675 kb)
Rights and permissions
About this article
Cite this article
Leone, L., Colussi, C., Gironi, K. et al. Altered Nup153 Expression Impairs the Function of Cultured Hippocampal Neural Stem Cells Isolated from a Mouse Model of Alzheimer’s Disease. Mol Neurobiol 56, 5934–5949 (2019). https://doi.org/10.1007/s12035-018-1466-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1466-1