Abstract
Background
Omalizumab, a therapeutic monoclonal antibody specific for human IgE, has thus far been used as add-on therapy for moderate-to-severe allergic asthma in adults and children.
Objective
The objective of this study was to test omalizumab efficacy in other conditions in which the IgE-mast cell axis is supposed to play a role.
Methods
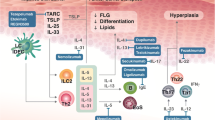
Nine patients with dermatological manifestations possibly related to activation of the IgE-mast cell axis (six chronic spontaneous urticaria and three atopic dermatitis patients) were administered off-label omalizumab because of refractoriness to standard therapy. All patients were subjected to strict clinical, laboratoristic, and imaging follow-up to monitor for possible adverse effects. In addition, to further assess the role of omalizumab on T cells, mast cells, and eosinophils, T-cell immune polarisation as well as eosinophil cationic protein and tryptase serum levels were determined before and during omalizumab administration.
Results
Therapy was effective in seven out of nine patients (six complete responses, one partial response, and two no responses). Interestingly, omalizumab appeared to induce lymphocyte polarisation toward a type 2 immune response and to be able to quench eosinophil-mediated inflammation, particularly in atopic dermatitis patients. Tryptase serum levels were generally low and remained unchanged during omalizumab treatment. Despite treatment spanning over several years in most of the patients, no adverse effects nor new ensuing medical conditions have thus far been observed (median follow-up: 42 months).
Conclusions
Off-label omalizumab was safe and effective in our patients. The novel immunologic features recorded in our patients add further complexity to the mechanism of action of omalizumab.





Similar content being viewed by others
References
Humbert N, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–16.
Chang TW, Wu PC, Hsu CL, Hung AF. Anti-IgE antibodies for the treatment of IgE-mediated allergic diseases. Adv Immunol. 2007;93:63–119.
Chang TW, Shiung YY. Anti-IgE as a mast cell-stabilizing therapeutic agent. J Allergy Clin Immunol. 2006;117:1203–13.
Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–76.
Tsabouri S, Tseretopoulou X, Priftis K, Ntzani EE. Omalizumab for the treatment of inadequately controlled allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. J Allergy Clin Immunol Pract. 2014;2:332–40.
Spector SL, Tan RA. Effect of omalizumab on patients with chronic urticaria. Ann Allergy Asthma Immunol. 2007;99:190–3.
Spector SL, Tan RA. Therapeutic alternatives for chronic urticaria: additional reports on omalizumab. Ann Allergy Asthma Immunol. 2008;101:647.
Vestergaard C, Deleuran M. Two cases of severe refractory chronic idiopathic urticaria treated with omalizumab. Acta Derm Venereol. 2010;90:443–4.
Romano C, Sellitto A, De Fanis U, Esposito G, Arbo P, Giunta R, Lucivero G. Maintenance of remission with low-dose omalizumab in long-lasting, refractory chronic urticaria. Ann Allergy Asthma Immunol. 2010;104:95–7.
Iemoli E, Piconi S, Fusi A, Borgonovo L, Borelli M, Trabattoni D. Immunological effects of omalizumab in chronic urticaria: a case report. J Investig Allergol Clin Immunol. 2010;20:252–4.
Kaplan AP, Joseph K, Maykut RJ, Geba GP, Zeldin RK. Treatment of chronic autoimmune urticaria with omalizumab. J Allergy Clin Immunol. 2008;122:569–73.
Ferrer M, Gamboa P, Sanz ML, Goikoetxea MJ, Cabrera-Freitag P, Javaloyes G, et al. Omalizumab is effective in nonautoimmune urticaria. J Allergy Clin Immunol. 2011;127:1300–2.
Saini S, Rosen KE, Hsieh HJ, Wong DA, Conner E, Kaplan A, et al. A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine-refractory chronic idiopathic urticaria. J Allergy Clin Immunol. 2011;128:567–73.
Maurer M, Altrichter S, Bieber T, Biedermann T, Brautigam M, Seyfried S, et al. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J Allergy Clin Immunol. 2011;128:202–9.
Maurer M, Rosen K, Hsieh HJ, Saini S, Grattan C, Gimenez-Arnau A, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368:924–35.
Kaplan A, Ledford D, Ashby M, Canvin J, Zazzali JL, Conner E, et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticariadespite standard combination therapy. J Allergy Clin Immunol. 2013;132:101–9.
Saavedra MC, Sur S. Down regulation of the high-affinity IgE receptor associated with successful treatment of chronic idiopathic urticaria with omalizumab. Clin Mol Allergy. 2011;9:2.
Asai K, Kitaura J, Kawakami Y, Yamagata N, Tsai M, Carbone DP, et al. Regulation of mast cell survival by IgE. Immunity. 2001;14:791–800.
Kalesnikoff J, Huber M, Lam V, Damen JE, Zhang J, Siraganian RP, et al. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 2001;14:801–11.
Altrichter S, Peter HJ, Pisarevskaja D, Metz M, Martus P, Maurer M. IgE mediated autoallergy against thyroid peroxidase—a novel pathomechanism of chronic spontaneous urticaria? PLoS One. 2011;6:e14794.
Hatada Y, Kashiwakura J, Hayama K, Fujisawa D, Sasaki-Sakamoto T, Terui T, et al. Significantly high levels of anti-dsDNA immunoglobulin E in sera and the ability of dsDNA to induce the degranulation of basophils from chronic urticaria patients. Int Arch Allergy Immunol. 2013;161(suppl 2):154–8.
Chang TW, Chen C, Lin C-J, Metz M, Church MK, Maurer M. The potential pharmacologic mechanisms of omalizumab in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2014. doi:10.1016/j.jaci.2014.04.036.
Morjaria JB, Polosa R. Off-label use of omalizumab in non-asthma conditions: new opportunities. Expert Rev Respir Med. 2009;3:299–308.
Incorvaia C, Mauro M, Russello M, Formigoni C, Riario-Sforza GG, Ridolo E. Omalizumab, an anti-immunoglobulin E antibody: state of the art. Drug Des Devel Ther. 2014;8:197–207.
Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69:868–87.
Eichenfield LF, Tom WL, Chamlin SL, Feldman SR, Hanifin JM, Simpson EL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338–51.
Jáuregui I, Ortiz de Frutos FJ, Ferrer M, Giménez-Arnau A, Sastre J, Bartra J, et al. Assessment of severity and quality of life in chronic urticaria. J Investig Allergol Clin Immunol. 2014;24:80–6.
Romano C, De Fanis U, Sellitto A, Chiurazzi F, Guastafierro S, Giunta R, et al. Induction of CD95 upregulation does not render chronic lymphocytic leukemia B-cells susceptible to CD95-mediated apoptosis. Immunol Lett. 2005;97:131–9.
Sellitto A, Galizia G, De Fanis U, Lieto E, Zamboli A, Orditura M, et al. Behavior of circulating CD4+CD25+Foxp3+ regulatory T cells in colon cancer patients undergoing surgery. J Clin Immunol. 2011;31:1095–104.
Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, et al. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–5.
Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–54.
Nagata K, Tanaka K, Ogawa K, Kemmotsu K, Imai T, Yoshie O, et al. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol. 1999;162:1278–86.
Cosmi L, Annunziato F, Iwasaki M, Galli G, Manetti R, Maggi E, et al. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur J Immunol. 2000;30:2972–9.
De Fanis U, Mori F, Kurnat RJ, Lee WK, Bova M, Adkinson NF, et al. GATA3 up-regulation associated with surface expression of CD294/CRTH2: a unique feature of human Th cells. Blood. 2007;109:4343–50.
Boin F, De Fanis U, Bartlett SJ, Wigley FM, Rosen A, Casolaro V. T cell polarization identifies distinct clinical phenotypes in scleroderma lung disease. Arthritis Rheum. 2008;58:1165–74.
De Fanis U, Wang GC, Fedarko NS, Walston JD, Casolaro V, Leng SX. T-lymphocytes expressing CC chemokine receptor-5 are increased in frail older adults. J Am Geriatr Soc. 2008;56:904–8.
Onoé K, Yanagawa Y, Minami K, Iijima N, Iwabuchi K. Th1 or Th2 balance regulated by interaction between dendritic cells and NKT cells. Immunol Res. 2007;38:319–32.
Iyengar SR, Hoyte EG, Loza A, Bonaccorso S, Chiang D, Umetsu DT, Nadeau KC. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162:89–93.
Baker BS. The role of microorganisms in atopic dermatitis. Clin Exp Immunol. 2006;144:1–9.
Furuta GT, Atkins FD, Lee NA, Lee JJ. Changing roles of eosinophils in health and disease. Ann Allergy Asthma Immunol. 2014;113:3–8.
Djukanovic R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. 2004;170:583–93.
Riccio AM, Dal Negro RW, Micheletto C, De Ferrari L, Folli C, Chiappori A, Canonica GW. Omalizumab modulates bronchial reticular basement membrane thickness and eosinophil infiltration in severe persistent allergic asthma patients. Int J Immunopathol Pharmacol. 2012;25:475–84.
Asero R, Cugno M, Tedeschi A. Eosinophils in chronic urticaria: supporting or leading actors? World Allergy Organ J. 2009;2:213–7.
Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;26:451–63.
Delong LK, Culler SD, Saini SS, Beck LA, Chen SC. Annual direct and indirect health care costs of chronic idiopathic urticaria: a cost analysis of 50 nonimmunosuppressed patients. Arch Dermatol. 2008;144:35–9.
Fowler JF, Duh MS, Rovba L, Buteau S, Pinheiro L, Lobo F, et al. The direct and indirect cost burden of atopic dermatitis: an employer-payer perspective. Manag Care Interface. 2007;20:26–32.
Conflict of interest
No sources of funding were used for the conduct of this study. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romano, C., Sellitto, A., De Fanis, U. et al. Omalizumab for Difficult-to-Treat Dermatological Conditions: Clinical and Immunological Features from a Retrospective Real-Life Experience. Clin Drug Investig 35, 159–168 (2015). https://doi.org/10.1007/s40261-015-0267-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-015-0267-9