Abstract
Background
The antiepileptic drug lacosamide has a low potential for drug–drug interactions, but is a substrate and moderate inhibitor of the cytochrome P450 (CYP) enzyme CYP2C19.
Objective
This phase I, randomized, open-label, two-way crossover trial evaluated the pharmacokinetic effects of lacosamide and omeprazole coadministration.
Methods
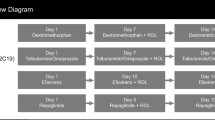
Healthy, White, male volunteers (n = 36) who were not poor metabolizers of CYP2C19 were randomized to treatment A (single-dose 40 mg omeprazole on days 1 and 8 together with 6 days of multiple-dose lacosamide [200–600 mg/day] on days 3–8) and treatment B (single doses of 300 mg lacosamide on days 1 and 8 with 7 days of 40 mg/day omeprazole on days 3–9) in pseudorandom order, separated by a ≥7-day washout period. Area under the concentration–time curve (AUC) and peak concentration (C max) were the primary pharmacokinetic parameters measured for lacosamide or omeprazole administered alone (reference) or in combination (test). Bioequivalence was determined if the 90 % confidence interval (CI) of the ratio (test/reference) fell within the acceptance range of 0.8–1.25.
Results
The point estimates (90 % CI) of the ratio of omeprazole + lacosamide coadministered versus omeprazole alone for AUC (1.098 [0.996–1.209]) and C max (1.105 [0.979–1.247]) fell within the acceptance range for bioequivalence. The point estimates (90 % CI) of the ratio of lacosamide + omeprazole coadministration versus lacosamide alone also fell within the acceptance range for bioequivalence (AUC 1.133 [1.102–1.165]); C max 0.996 (0.947–1.047).
Conclusion
Steady-state lacosamide did not influence omeprazole single-dose pharmacokinetics, and multiple-dose omeprazole did not influence lacosamide single-dose pharmacokinetics.



Similar content being viewed by others
References
Zanger UM, Turpeinen M, Klein K, et al. Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal Bioanal Chem. 2008;392:1093–108.
US Food and Drug Administration (FDA). Drug Development and Drug Interactions. 2011 16 Sept 2011 [cited 2013 April 18th]. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm080499.htm.
Flockhart DA. Drug Interactions: Cytochrome P450 Drug Interaction Table. Indiana University School of Medicine (2007). 2007 [cited 2014 Jan 20th]. http://medicine.iupui.edu/clinpharm/ddis/table.aspx.
Li-Wan-Po A, Girard T, Farndon P, et al. Pharmacogenetics of CYP2C19: functional and clinical implications of a new variant CYP2C19*17. Br J Clin Pharmacol. 2010;69:222–30.
Levy RH. Cytochrome P450 isozymes and antiepileptic drug interactions. Epilepsia. 1995;36(Suppl 5):S8–13.
Meyer UA. Interaction of proton pump inhibitors with cytochromes P450: consequences for drug interactions. Yale J Biol Med. 1996;69:203–9.
Lakehal F, Wurden CJ, Kalhorn TF, et al. Carbamazepine and oxcarbazepine decrease phenytoin metabolism through inhibition of CYP2C19. Epilepsy Res. 2002;52:79–83.
Preissner S, Kroll K, Dunkel M, et al. SuperCYP: a comprehensive database on Cytochrome P450 enzymes including a tool for analysis of CYP–drug interactions. Nucl Acids Res. 2010;38:D237–43.
UCB Pharma. Vimpat® (lacosamide) EPAR Product Information. 2013 [EU Prescribing Information]. Brussels: UCB Pharma SA.
UCB Inc. Vimpat® (lacosamide tablets, injection, oral solution) [U.S. prescribing information]. Smyrna: UCB Inc.
Cawello W, Boekens H, Bonn R. Absorption, disposition, metabolic fate and elimination of the anti-epileptic drug lacosamide in humans: mass balance following intravenous and oral administration. Eur J Drug Metab Pharmacokinet. 2012;37:241–8.
Cawello W, Bonn R. No pharmacokinetic interaction between lacosamide and valproic acid in healthy volunteers. J Clin Pharmacol. 2012;52:1739–48.
Cawello W, Nickel B, Eggert-Formella A. No pharmacokinetic interaction between lacosamide and carbamazepine in healthy volunteers. J Clin Pharmacol. 2010;50:459–71.
Cawello W, Rosenkranz B, Schmid B, et al. Pharmacodynamic and pharmacokinetic evaluation of coadministration of lacosamide and an oral contraceptive (levonorgestrel plus ethinylestradiol) in healthy female volunteers. Epilepsia. 2013;54:530–6.
Thomas D, Scharfenecker U, Nickel B, et al. Lacosamide has low potential for drug–drug interaction. Epilepsia. 2008;50:110 [abstract #T232].
Beyreuther BK, Freitag J, Heers C, et al. Lacosamide: a review of preclinical properties. CNS Drug Rev. 2007;13:21–42.
Cawello W, Andeas J-O, Hebert D, et al. Pharmacokinetic evaluation of oral lacosamide in Phase II/III clinical trials: a pooled analysis [abstract]. Epilepsia. 2010;51(Suppl. 4):68.
Hoy SM. Lacosamide: a review of its use as adjunctive therapy in the management of partial-onset seizures. CNS Drugs. 2013;27:1125–42.
Fellenius E, Berglindh T, Sachs G, et al. Substituted benzimidazoles inhibit gastric acid secretion by blocking (H+ + K+)ATPase. Nature. 1981;290:159–61.
Stedman CA, Barclay ML. Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors. Aliment Pharmacol Ther. 2000;14:963–78.
Andersson T, Miners JO, Veronese ME, et al. Identification of human liver cytochrome P450 isoforms mediating omeprazole metabolism. Br J Clin Pharmacol. 1993;36:521–30.
Omeprazol-ratiopharm® NT. Summary of Product Characteristics (Fachinformation), ratiopharm GmbH, September (2004). [cited 2014 Feb 18th]. http://www.imb.ie/images/uploaded/swedocuments/2134427.PA0749_092_001.c80ae5fa-b291-47ea-97e4-fb4c4f741234.000001Omeprazole%20PIL.131017.pdf
Zevin S, Benowitz NL. Drug interactions with tobacco smoking. An update. Clin Pharmacokinet. 1999;36:425–38.
Zakhari S. Overview: how is alcohol metabolized by the body? [cited 2014 Jan 20th]. http://pubs.niaaa.nih.gov/publications/arh294/245-255.htm.
Tassaneeyakul W, Birkett DJ, McManus ME, et al. Caffeine metabolism by human hepatic cytochromes P450: contributions of 1A2, 2E1 and 3A isoforms. Biochem Pharmacol. 1994;47:1767–76.
European Medicines Agency. The European Agency for the Evaluation of Medicinal Products, Committee for Proprietary Medicinal Products (CPMP). Note for guidance on the investigation of bioavailability and bioequivalence (CPMP/EWP/QWP/1401/98). London, 2001. [cited 2014 Feb 18th]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003008.pdf
US Food and Drug Administration (FDA). Guidance for industry: bioavailability and bioequivalence studies for orally administered drug products—general considerations. Center for Drug Evaluation and Research; Draft July 2002: Rockville; 2002.
Cawello W, Bökens O, Nickel B, et al. Tolerability, pharmacokinetics, and bioequivalence of the tablet and syrup formulations of lacosamide in plasma, saliva, and urine: saliva as a surrogate of pharmacokinetics in the central compartment. Epilepsia. 2013;54:81–8.
Acknowledgments
The authors thank Pharm PlanNet Contract Research GmbH (Mönchengladbach, Germany) for performing the clinical part of the trial, in accordance with the current laws of Germany, and Independent Clinical Research Consulting (Berlin, Germany) for data management. We further thank IKP Bobenheim (Grünstadt, Germany) for the analytical work on both lacosamide and omeprazole samples and Medizinische Laboratorien Marienhof GmbH (Mönchengladbach, Germany) for the safety laboratory analyses. Biostatistical analysis was performed by the CRO M.A.R.C.O. Institute for Clinical Research and Statistics—Dr. Wargenau (Düsseldorf, Germany) under the supervision of UCB Pharma. UCB Pharma was responsible for clinical trial supply management and also helped supervise the clinical trial. Merrilee Johnstone, PhD, from Prescott Medical Communications Group (Chicago, IL, USA) provided writing assistance. Editorial support was provided by Karen Burrows of Evidence Scientific Solutions (Horsham, UK), which was funded by UCB Pharma.
Financial Disclosure
UCB Pharma (Monheim, Germany) provided the trial supplies and sponsored and funded the trial. The authors are employees of UCB Biosciences GmbH (part of UCB Pharma).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cawello, W., Mueller-Voessing, C. & Fichtner, A. Pharmacokinetics of Lacosamide and Omeprazole Coadministration in Healthy Volunteers: Results from a Phase I, Randomized, Crossover Trial. Clin Drug Investig 34, 317–325 (2014). https://doi.org/10.1007/s40261-014-0177-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-014-0177-2