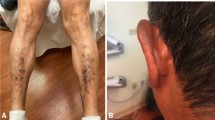
Abstract
Eczematous drug eruptions are a heterogenous group of skin reactions that resemble eczema both clinically and histologically. We reviewed the literature and cataloged the systemically administered medications that cause these eruptions, along with their characteristic clinical presentations. We identified three primary pathophysiologic etiologies: (1) cutaneous immunomodulation, (2) skin dehydration, and (3) delayed hypersensitivity. Notably, eczematous eruptions caused by altered immunity in the skin may be increasing in incidence as some responsible drugs, in particular biologic therapies (such as tumor necrosis factor-α and interleukin-17 inhibitors) and targeted cancer treatments (including immune checkpoint inhibitors and epidermal growth factor receptor inhibitors), become more commonly employed in clinical practice. Other notable causes of eczematous eruptions include antiviral agents for hepatitis C virus and cardiovascular medications in elderly individuals, and notable subtypes of eczematous reactions include systemic contact dermatitis and photoallergic reactions, which are also discussed. The diagnostic gold standard is drug rechallenge and most reactions may be treated effectively with emollients, topical corticosteroids, and oral antihistamines.



Similar content being viewed by others
References
Apaydin R, Dökmeci Ş, Bayramgürler D, Yildirim G. Drug eruptions: a study including all inpatients and outpatients at a dermatology clinic of a university hospital. J Eur Acad Dermatol Venereol. 2000;14:518–20.
Barbaud A, Reichert-Penetrat S, Tréchot P, Jacquin-Petit MA, Ehlinger A, Noirez V, et al. The use of skin testing in the investigation of cutaneous adverse drug reactions. Br J Dermatol. 1998;139:49–58.
Borch JE, Andersen KE, Bindslev-Jensen C. The prevalence of acute cutaneous drug reactions in a Scandinavian university hospital. Acta Derm Venereol. 2006;86:518–22.
Segaert S, Hermans C. Clinical signs, pathophysiology and management of cutaneous side effects of anti-tumor necrosis factor agents. Am J Clin Dermatol. 2017;18:771–87.
Cossio M-L, Genois A, Jantchou P, Hatami A, Deslandres C, McCuaig C. Skin manifestations in pediatric patients treated with a TNF-alpha inhibitor for inflammatory bowel disease: a retrospective study. J Cutan Med Surg. 2020;24(4):333–9.
Nakamura M, Lee K, Singh R, Zhu TH, Farahnik B, Abrouk M, et al. Eczema as an adverse effect of anti-TNFα therapy in psoriasis and other Th1-mediated diseases: a review. J Dermatol Treat. 2017;28:237–41.
Tillack C, Ehmann LM, Friedrich M, Laubender RP, Papay P, Vogelsang H, et al. Anti-TNF antibody-induced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-γ-expressing Th1 cells and IL-17A/IL-22-expressing Th17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut. 2014;63:567–77.
Al-Janabi A, Foulkes AC, Mason K, Smith CH, Grif CEM. Phenotypic switch to eczema in patients receiving biologics for plaque psoriasis: a systematic review. J Eur Acad Dermatol Venereol. 2020;34:1440–8.
Esmailzadeh A, Yousefi P, Farhi D, Bachmeyer C, Cosnes J, Berenbaum F, et al. Predictive factors of eczema-like eruptions among patients without cutaneous psoriasis receiving infliximab: a cohort study of 92 patients. Dermatology (Basel). 2009;219:263–7.
Soh JS, Yun WJ, Kim K-J, Won CH, Park SH, Yang D-H, et al. Concomitant use of azathioprine/6-mercaptopurine decreases the risk of anti-TNF-induced skin lesions. Inflamm Bowel Dis. 2015;21:832–9.
Ezzedine K, Visseaux L, Cadiot G, Brixi H, Bernard P, Reguiai Z. Ustekinumab for skin reactions associated with anti-tumor necrosis factor-α agents in patients with inflammatory bowel diseases: a single-center retrospective study. J Dermatol. 2019;46:322–7.
Afzali A, Wheat CL, Hu JK, Olerud JE, Lee SD. The association of psoriasiform rash with anti-tumor necrosis factor (anti-TNF) therapy in inflammatory bowel disease: a single academic center case series. J Crohns Colitis. 2014;8:480–8.
Garcovich S, De Simone C, Genovese G, Berti E, Cugno M, Marzano AV. Paradoxical skin reactions to biologics in patients with rheumatologic disorders. Front Pharmacol. 2019;10:282.
Cleynen I, Van Moerkercke W, Billiet T, Vandecandelaere P, Vande Casteele N, Breynaert C, et al. Characteristics of skin lesions associated with anti-tumor necrosis factor therapy in patients with inflammatory bowel disease. Ann Intern Med. 2015;164:10–22.
Seneschal J, Milpied B, Vergier B, Lepreux S, Schaeverbeke T, Taïeb A. Cytokine imbalance with increased production of interferon-α in psoriasiform eruptions associated with antitumour necrosis factor-α treatments. Br J Dermatol. 2009;161:1081–8.
Succaria F, Bhawan J. Cutaneous side-effects of biologics in immune-mediated disorders: a histopathological perspective. J Dermatol. 2017;44:243–50.
Stoffel E, Maier H, Riedl E, Brüggen M-C, Reininger B, Schaschinger M, et al. Analysis of anti-tumour necrosis factor-induced skin lesions reveals strong T helper 1 activation with some distinct immunological characteristics. Br J Dermatol. 2018;178:1151–62.
Conrad C, Di Domizio J, Mylonas A, Belkhodja C, Demaria O, Navarini AA, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25.
Ariyasu T, Tanaka T, Fujioka N, Yanai Y, Yamamoto S, Yamauchi H, et al. Effects of interferon-alpha subtypes on the TH1/TH2 balance in peripheral blood mononuclear cells from patients with hepatitis virus infection-associated liver disorders. Vitro Cell Dev Biol Anim. 2005;41:50–6.
Poreaux C, Bronowicki J-P, Debouverie M, Schmutz J-L, Waton J, Barbaud A. Managing generalized interferon-induced eruptions and the effectiveness of desensitization. Clin Exp Allergy. 2014;44:756–64.
Hawkes JE, Yan BY, Chan TC, Krueger JG. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201:1605–13.
Reddy V, Yang EJ, Myers B, Liao W. Clinical evaluation of risankizumab-rzaa in the treatment of plaque psoriasis. J Inflamm Res. 2020;13:53–60.
Napolitano M, Megna M, Fabbrocini G, Nisticò SP, Balato N, Dastoli S, et al. Eczematous eruption during anti-interleukin 17 treatment of psoriasis: an emerging condition. Br J Dermatol. 2019;181:604–6.
Saeki H, Nakagawa H, Nakajo K, Ishii T, Morisaki Y, Aoki T, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44:355–62.
Caldarola G, Pirro F, Di Stefani A, Talamonti M, Galluzzo M, D’Adamio S, et al. Clinical and histopathological characterization of eczematous eruptions occurring in course of anti IL-17 treatment: a case series and review of the literature. Expert Opin Biol Ther. 2020;20:665–72.
Munera-Campos M, Ballesca F, Richarz N, Ferrandiz C, Carrascosa JM. Paradoxical eczematous reaction to ixekizumab. J Eur Acad Dermatol Venereol. 2019;33:e40–2.
Reyn B, Hillary T, Gils A. Eczematous eruption after guselkumab treatment for psoriasis. JAAD Case Rep. 2019;5:973–5.
Chicharro P, Rodríguez-Jiménez P, De la Fuente H, Fraga-Fernández J, Cibrian D, Sánchez-Madrid F, et al. Mixed profile of cytokines in paradoxical eczematous eruptions associated with anti-IL-17 therapy. J Allergy Clin Immunol Pract. 2020;8:3619–21.
Luchsinger I, Knöpfel N, Theiler M, Claustres MB, Barbieux C, Schwieger-Briel A, et al. Secukinumab therapy for Netherton syndrome. JAMA Dermatol. 2020;156:907–11.
Napolitano M, Gallo L, Patruno C, Fabbrocini G, Megna M. Eczematous reaction to ixekizumab successfully treated with dupilumab. Dermatol Ther. 2020;33:e13218.
Galluzzo M, D’Adamio S, Massaro A, Piccolo A, Bianchi L, Talamonti M. Spotlight on brodalumab in the treatment of plaque psoriasis: the evidence to date. Clin Cosmet Investig Dermatol. 2019;12:311–21.
Cullison SRJ, Siegfried EC. Paradoxic eczema in infants after heart transplantation. Pediatr Dermatol. 2018;35:e29-34.
Bumbacea RS, Ghiordanescu I. Atopic dermatitis induced by systemic immunosuppression with tacrolimus-case report. Eur Ann Allergy Clin Immunol. 2013;45:109–10.
Saito R, Sawada Y, Nakamura M. Two cases of eczematous drug eruption caused by oral tacrolimus administration. Contact Dermat. 2017;77:128–30.
Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11:3801.
Sibaud V. Dermatologic reactions to immune checkpoint inhibitors. Am J Clin Dermatol. 2018;19:345–61.
Chan L, Hwang SJE, Byth K, Kyaw M, Carlino MS, Chou S, et al. Survival and prognosis of individuals receiving programmed cell death 1 inhibitor with and without immunologic cutaneous adverse events. J Am Acad Dermatol. 2020;82:311–6.
Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206–12.
Geisler AN, Phillips GS, Barrios DM, Wu J, Leung DYM, Moy AP, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255–68.
Bottlaender L, Amini-Adle M, Maucort-Boulch D, Robinson P, Thomas L, Dalle S. Cutaneous adverse events: a predictor of tumour response under anti-PD-1 therapy for metastatic melanoma, a cohort analysis of 189 patients. J Eur Acad Dermatol Venereol. 2020;34:2096–105.
Gnanendran SS, Turner LM, Miller JA, Hwang SJE, Miller AC. Cutaneous adverse events of anti-PD-1 therapy and BRAF inhibitors. Curr Treat Options Oncol. 2020;21:29.
Lee YJ, Kim HT, Won CH, Chang SE, Lee MW, Choi JH, et al. Characterization and prognostic significance of cutaneous adverse events to anti-programmed cell death-1 therapy. J Korean Med Sci. 2019;34:e186.
Coleman E, Ko C, Dai F, Tomayko MM, Kluger H, Leventhal JS. Inflammatory eruptions associated with immune checkpoint inhibitor therapy: a single-institution retrospective analysis with stratification of reactions by toxicity and implications for management. J Am Acad Dermatol. 2019;80:990–7.
Welborn M, Kubicki SL, Garg N, Patel AB. Retrospective chart review of cutaneous adverse events associated with tremelimumab in 17 patients. Am J Clin Dermatol. 2018;19:899–905.
Hwang SJE, Carlos G, Wakade D, Byth K, Kong BY, Chou S, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74(455–61):e1.
Belum VR, Benhuri B, Postow MA, Hellmann MD, Lesokhin AM, Segal NH, et al. Characterization and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25.
Sibaud V, Meyer N, Lamant L, Vigarios E, Mazieres J, Delord JP. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254–63.
Kaunitz GJ, Loss M, Rizvi H, Ravi S, Cuda JD, Bleich KB, et al. Cutaneous eruptions in patients receiving immune checkpoint blockade: clinicopathologic analysis of the nonlichenoid histologic pattern. Am J Surg Pathol. 2017;41:1381–9.
Min Lee CK, Li S, Tran DC, Zhu GA, Kim J, Kwong BY, et al. Characterization of dermatitis after PD-1/PD-L1 inhibitor therapy and association with multiple oncologic outcomes: a retrospective case-control study. J Am Acad Dermatol. 2018;79:1047–52.
Lacouture M, Sibaud V. Toxic side effects of targeted therapies and immunotherapies affecting the skin, oral mucosa, hair, and nails. Am J Clin Dermatol. 2018;19:31–9.
Ricciardi S, Tomao S, de Marinis F. Toxicity of targeted therapy in non-small-cell lung cancer management. Clin Lung Cancer. 2009;10:28–35.
Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6:803–12.
Hu JC, Sadeghi P, Pinter-Brown LC, Yashar S, Chiu MW. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56:317–26.
Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16:1425–33.
Ocvirk J, Cencelj S. Management of cutaneous side-effects of cetuximab therapy in patients with metastatic colorectal cancer. J Eur Acad Dermatol Venereol. 2010;24:453–9.
Štulhofer Buzina D, Martinac I, Ledić Drvar D, Čeović R, Bilić I, Marinović B. Adverse reaction to cetuximab, an epidermal growth factor receptor inhibitor. Acta Dermatovenerol Croat. 2016;24:70–2.
Wnorowski AM, de Souza A, Chachoua A, Cohen DE. The management of EGFR inhibitor adverse events: a case series and treatment paradigm. Int J Dermatol. 2012;51:223–32.
Barbaud A, Granel F, Waton J, Poreaux C. How to manage hypersensitivity reactions to biological agents? Eur J Dermatol. 2011;21:667–74.
Chiu HY, Cheng YP, Jee SH, Tsai TF. Erlotinib-induced generalized eczema craquelé and follicular rash sparing the previous radiation field. Clin Exp Dermatol. 2012;37:922–4.
Lichtenberger BM, Gerber PA, Holcmann M, Buhren BA, Amberg N, Smolle V, et al. Epidermal EGFR controls cutaneous host defense and prevents inflammation. Sci Transl Med. 2013;5:199ra111.
Albanell J, Rojo F, Averbuch S, Feyereislova A, Mascaro JM, Herbst R, et al. Pharmacodynamic studies of the epidermal growth factor receptor inhibitor ZD1839 in skin from cancer patients: histopathologic and molecular consequences of receptor inhibition. J Clin Oncol. 2002;20:110–24.
Sankhala K, Mita A, Kelly K, Mahalingam D, Giles F, Mita M. The emerging safety profile of mTOR inhibitors, a novel class of anticancer agents. Target Oncol. 2009;4:135–42.
Habu M, Tohyama M, Sayama K. Two cases of eczematous eruptions caused by everolimus. J Cutan Immunol Allergy. 2019;2:135–8.
Shameem R, Lacouture M, Wu S. Incidence and risk of rash to mTOR inhibitors in cancer patients: a meta-analysis of randomized controlled trials. Acta Oncol. 2015;54:124–32.
Balagula Y, Rosen A, Tan BH, Busam KJ, Pulitzer MP, Motzer RJ, et al. Clinical and histopathologic characteristics of rash in cancer patients treated with mammalian target of rapamycin inhibitors. Cancer. 2012;118:5078–83.
Raymond E, Alexandre J, Faivre S, Vera K, Materman E, Boni J, et al. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol. 2004;22:2336–47.
Frouin E, Sebille G, Freudenberger S, Menecier B, Quoix E, Criber B, et al. Asteatotic eczema (‘eczema craquelé’) with histopathological interface dermatitis: a new cutaneous reaction following pemetrexed. Br J Dermatol. 2012;166:1359–60.
Hazan E, Santa E, Sahu J. Diffuse rash after the administration of carboplatin and paclitaxel: answer. Am J Dermatopathol. 2016;38:393.
Brandt K, Schäkel K, Stölzel F, Janschek J, Ehninger G, Schaich M. Auricular oedema and dyshidrotic eczema in a patient with acute myeloid leukaemia treated with cytarabine. Case Rep Oncol. 2010;3:349–53.
Fluhr JW, Miteva M, Primavera G, Ziemer M, Elsner P, Berardesca E. Functional assessment of a skin care system in patients on chemotherapy. Skin Pharmacol Physiol. 2007;20:253–9.
Roujeau J-C, Mockenhaupt M, Tahan SR, Henshaw J, Martin EC, Harding M, et al. Telaprevir-related dermatitis. JAMA Dermatol. 2013;149:152–8.
Patel P, Malik K, Krishnamurthy K. Cutaneous adverse events in chronic hepatitis C patients treated with new direct-acting antivirals: a systematic review and meta-analysis. J Cutan Med Surg. 2016;20:58–66.
Tavakoli-Tabasi S. A Longitudinal cohort study of mucocutaneous drug eruptions during interferon and ribavirin treatment of hepatitis C. J Clin Gastroenterol. 2012;46:6.
Vázquez-López F, Manjón-Haces JA, Pérez-Alvarez R, Pérez-Oliva N. Eczema-like lesions and disruption of therapy in patients treated with interferon-alfa and ribavirin for chronic hepatitis C: the value of an interdisciplinary assessment. Br J Dermatol. 2004;150:1046–7.
Patrk I, Morović M, Markulin A, Patrk J. Cutaneous reactions in patients with chronic hepatitis C treated with peginterferon and ribavirin. Dermatology. 2014;228:42–6.
Li Z, Zhang Y, An J, Feng Y, Deng H, Xiao S, et al. Predictive factors for adverse dermatological events during pegylated/interferon alpha and ribavirin treatment for hepatitis C. J Clin Virol. 2014;60:190–5.
Garcias-Ladaria J, Pérez-Ferriols A, Ortega-García P, Diago M. Management of refractory telaprevir-induced dermatitis using oral corticosteroids. Actas Dermosifiliogr. 2014;105:e55-60.
Cacoub P, Bourlière M, Lübbe J, Dupin N, Buggisch P, Dusheiko G, et al. Dermatological side effects of hepatitis C and its treatment: patient management in the era of direct-acting antivirals. J Hepatol. 2012;56:455–63.
Carrascosa R, Capusan T-M, Llamas-Velasco M, García-Buey L, Gordillo C, Sánchez-Pérez J. High frequency of severe telaprevir-associated skin eruptions in clinical practice. Acta Derm Venereol. 2016;96:97–9.
Guillot B, Blazquez L, Bessis D, Dereure O, Guilhou JJ. A prospective study of cutaneous adverse events induced by low-dose alpha-interferon treatment for malignant melanoma. Dermatology. 2004;208:49–54.
Kappos L, Wiendl H, Selmaj K, Arnold DL, Havrdova E, Boyko A, et al. Daclizumab HYP versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2015;373:1418–28.
Mistry N, Shapero J, Crawford RI. A review of adverse cutaneous drug reactions resulting from the use of interferon and ribavirin. Can J Gastroenterol. 2009;23:677–83.
Dereure O, Raison-Peyron N, Larrey D, Blanc F, Guilhou J-J. Diffuse inflammatory lesions in patients treated with interferon alfa and ribavirin for hepatitis C: a series of 20 patients. Br J Dermatol. 2002;147:1142–6.
do Vale EPBM, Rodrigues CHL, Kallas FE, Fucuta PS. Eczema craquelé associated with antiviral treatment for chronic hepatitis C. An Bras Dermatol. 2017;92:436–7.
Moore MM, Elpern DJ, Carter DJ. Severe, generalized nummular eczema secondary to interferon alfa-2b plus ribavirin combination therapy in a patient with chronic hepatitis C virus infection. Arch Dermatol. 2004;140:215–7.
Shen Y, Pielop J, Hsu S. Generalized nummular eczema secondary to peginterferon alfa-2b and ribavirin combination therapy for hepatitis C infection. Arch Dermatol. 2005;141:102–3.
Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, Bea-Ardebol S. Eczematous drug eruption induced by sofosbuvir/velpatasvir: the need for a better classification. Am J Ther. 2019. https://doi.org/10.1097/MJT.0000000000001071.
Heng YK, Lim YL. Cutaneous adverse drug reactions in the elderly. Curr Opin Allergy Clin Immunol. 2015;15:300–7.
Young JWS. Cutaneous drug reactions in the elderly. Drugs Aging. 2017;34(9):655–72.
Mekić S, Jacobs LC, Gunn DA, Mayes AE, Ikram MA, Pardo LM, et al. Prevalence and determinants for xerosis cutis in the middle-aged and elderly population: a cross-sectional study. J Am Acad Dermatol. 2019;81(963–9):e2.
Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin. Am J Clin Dermatol. 2009;10:73–86.
Joly P, Benoit-Corven C, Baricault S, Lambert A, Hellot MF, Josset V, et al. Chronic eczematous eruptions of the elderly are associated with chronic exposure to calcium channel blockers: results from a case-control study. J Invest Dermatol. 2007;127:2766–71.
Summers EM, Bingham CS, Dahle KW, Sweeney C, Ying J, Sontheimer RD. Chronic eczematous eruptions in the aging: further support for an association with exposure to calcium channel blockers. JAMA Dermatol. 2013;149:814–8.
Baccino D, Merlo G, Cozzani E, Rosa GM, Tini G, Burlando M, et al. Cutaneous effects of antihypertensive drugs. G Ital Dermatol E Venereol. 2020;155:202–11.
Yoo J, Jue M-S. Intractable pruritus with chronic eczema in an elderly patient caused by long-term intake of calcium channel blocker. Contact Dermat. 2017;77:339–40.
Summers EM, Blickenstaff NR, Coman GC, Martins TB, Hill HR, Sontheimer RD. A pilot study evaluating biomarker development for drug-induced chronic eczematous eruptions of aging individuals. Ann Transl Med. 2017;5:393.
Özkaya E, Yazganoğlu KD. Angiotensin-converting enzyme inhibitors. In: Özkaya E, Yazganoğlu KD, editors. Adverse cutaneous drug reactions to cardiovascular drugs. London: Springer; 2014. p. 67–83.
Özkaya E, Yazganoğlu KD. Angiotensin II receptor blockers. In: Özkaya E, Yazganoğlu KD, editors. Adverse cutaneous drug reactions to cardiovascular drugs. London: Springer; 2014. p. 85–92.
Vena GA, Cassano N, Coco V, De Simone C. Eczematous reactions due to angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers. Immunopharmacol Immunotoxicol. 2013;35:447–50.
Robinson HN, Morison WL, Hood AF. Thiazide diuretic therapy and chronic photosensitivity. Arch Dermatol. 1985;121:522–4.
Thestrup-Pedersen K. Adverse reactions in the skin from anti-hypertensive drugs. Dan Med Bull. 1987;34(Suppl. 1):3–5.
Church R. Eczema provoked by methyl dopa. Br J Dermatol. 1974;91:373–8.
Heid E, Samsoen M, Juillard J, Eberst E, Foussereau J. Papulo-vesicular endogenous eruptions induced by methyldopa and clofibrate (author’s transl). Ann Dermatol Venereol. 1977;104:494–6.
Cheung K, Powers EM, McKillip J, Powers JG. Effect of statin use on incidence of eczema and atopic dermatitis: a retrospective cohort study. J Am Acad Dermatol. 2020. https://doi.org/10.1016/j.jaad.2020.05.015.
Krasovec M, Elsner P, Burg G. Generalized eczematous skin rash possibly due to HMG-CoA reductase inhibitors. Dermatology. 1993;186:248–52.
Feldmann R, Mainetti C, Saurat J-H. Skin lesions due to treatment with simvastatin (Zocor®). Dermatology. 1993;186:272.
de Boer EM, Bruynzeel DP. Allergy to pravastatin. Contact Dermat. 1994;30:238.
Salna MP, Singer HM, Dana AN. Pravastatin-induced eczematous eruption mimicking psoriasis. Case Rep Dermatol Med. 2017;2017:3418204.
Jowkar F, Namazi MR. Statins in dermatology. Int J Dermatol. 2010;49:1235–43.
Gerstenblith MR, Antony AK, Junkins-Hopkins JM, Abuav R. Pompholyx and eczematous reactions associated with intravenous immunoglobulin therapy. J Am Acad Dermatol. 2012;66:312–6.
Yokoyama K, Yoshida A. Late-onset and long-term systemic dyshidrotic eczema after intravenous immunoglobulin treatment for Kawasaki disease. BMJ Case Rep. 2019;12:e229596.
Shiraishi T, Yamamoto T. Severe dyshidrotic eczema after intravenous immunoglobulin therapy for Kawasaki syndrome. Pediatr Dermatol. 2013;30:e30–1.
Brazzelli V, Grassi S, Savasta S, Ruffinazzi G, Carugno A, Barbaccia V, et al. Pompholyx of the hands after intravenous immunoglobulin therapy for clinically isolated syndrome: a paediatric case. Int J Immunopathol Pharmacol. 2014;27:127–30.
Sarmiento E, Micheloud D, Carbone J. Complement consumption associated with eczematous cutaneous reaction during infusions of high doses of intravenous immunoglobulin. Br J Dermatol. 2004;151:721.
Kotan D, Erdem T, Acar BA, Boluk A. Dyshidrotic eczema associated with the use of IVIg. BMJ Case Rep. 2013. https://doi.org/10.1136/bcr-2012-008001.
Berk-Krauss J, Lee K, Lo Sicco KI, Liebman TN. Eczematous reaction to IVIG for the treatment of dermatomyositis. Int J Womens Dermatol. 2018;4:170–3.
Lee KC, Ladizinski B. Dyshidrotic eczema following intravenous immunoglobulin treatment. Can Med Assoc J. 2013;185:E530.
Miyamoto J, Böckle BC, Zillikens D, Schmidt E, Schmuth M. Eczematous reaction to intravenous immunoglobulin: an alternative cause of eczema. JAMA Dermatol. 2014;150:1120–2.
Vecchietti G, Kerl K, Prins C, Kaya G, Saurat J-H, French LE. Severe eczematous skin reaction after high-dose intravenous immunoglobulin infusion: report of 4 cases and review of the literature. Arch Dermatol. 2006;142:213–7.
Perez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139:S1-46.
Özkaya-Bayazit E, Güngör H. Carbamazepine induced eczematous eruptionclinically resembling atopic dermatitis. J Eur Acad Dermatol Venereol. 1999;12:182–3.
Terui T, Tagami H. Eczematous drug eruption from carbamazepine: coexistence of contact and photocontact sensitivity. Contact Dermat. 1989;20:260–4.
Roberts DL, Marks R. Skin reactions to carbamazepine. Arch Dermatol. 1981;117:273–5.
Akpinar F, Dervis E. Drug eruptions: an 8-year study including 106 inpatients at a dermatology clinic in Turkey. Indian J Dermatol. 2012;57:194–8.
Pigatto PD, Morelli M, Polenghi MM, Mozzanica N, Altomare GF. Phenobarbital-induced allergic dermatitis. Contact Dermat. 1987;16:279.
Sanz-Navarro J, Feal C, Dauden E. Treatment of human scabies with oral ivermectin: eczematous eruptions as a new non-reported adverse event. Actas Dermosifiliogr. 2017;108:643–9.
Nakatani K, Kurose T, Hyo T, Watanabe K, Yabe D, Kawamoto T, et al. Drug-induced generalized skin eruption in a diabetes mellitus patient receiving a dipeptidyl peptidase-4 inhibitor plus metformin. Diabetes Ther. 2012;3:14.
Guliani A, Yadav TC, Jha K. Type 2 diabetic female with vildagliptin associated eczematous eruption. Postgrad Med J. 2019;95:392–3.
Sugiyama S, Tanaka R, Hayashi H, Izumi K, Nishie W, Aoyama Y. Acquired haemophilia A in DPP4 inhibitor-induced bullous pemphigoid as immune reconstitution syndrome. Acta Derm Venereol. 2020;100:adv00178.
Tripathy A, Kumari KM, Babu AVM, Pai SB, Kumar DM. Anything rare is possible: letrozole induced eczematous skin eruption. J Clin Diagn Res. 2014;8:YD03-4.
Yoshida A, Sugita K, Tani N, Yamamoto O. Correlation between serum thymus and activation-regulated chemokine levels and eczematous drug eruption following oral challenge test with clonazepam. Clin Exp Dermatol. 2020;45:1063–5.
Harowick N, King CM. Generalized eczematous reaction to erythropoietin. Contact Dermat. 1993;28:123.
Salava A, Alanko K, Hyry H. Dipyridamole-induced eczematous drug eruption with positive patch test reaction. Contact Dermat. 2012;67:103–4.
Saito-Sasaki N, Sawada Y, Nishio D, Nakamura M. First case of drug eruption due to ipragliflozin: case report and review of the literature. Australas J Dermatol. 2017;58:236–8.
Kim DH, Koo DW, Jung KE, Lee JS. An eczematous drug eruption caused by methylsulfonylmethane. Australas J Dermatol. 2016;57:e149–50.
Winnicki M, Shear NH. A systematic approach to systemic contact dermatitis and symmetric drug-related intertriginous and flexural exanthema (SDRIFE): a closer look at these conditions and an approach to intertriginous eruptions. Am J Clin Dermatol. 2011;12:171–80.
Aquino M, Rosner G. Systemic contact dermatitis. Clin Rev Allergy Immunol. 2019;56:9–18.
Veien NK. Systemic contact dermatitis. Int J Dermatol. 2011;50:1445–56.
Angelini G, Vena GA, Grandolfo M, Mastrolonardo M. Iatrogenic contact dermatitis and eczematous reactions. Clin Dermatol. 1993;11:467–77.
Fowler JF. Systemic contact dermatitis caused by oral chromium picolinate. Cutis. 2000;65:116.
Skog E. Systemic eczematous contact-type dermatitis induced by iodochlorhydroxyquin and chloroquine phosphate. Contact Dermat. 1975;1:187.
Özkaya E, Topkarci Z, Özarmaǧan G. Systemic allergic dermatitis from chromium in a multivitamin/multimineral tablet. Contact Dermat. 2010;62:184.
Lawrence CM, Byrne JPH. Eczematous eruption from oral diphenhydramine. Contact Dermat. 1981;7:276–7.
Poon E, Fewings JM. Generalized eczematous reaction to budesonide in a nasal spray with cross-reactivity to triamcinolone. Australas J Dermatol. 2001;42:36–7.
Tomb RR, Lepoittevin J-P, Espinassouze F, Heid E, Foussereau J. Systemic contact dermatitis from pseudoephedrine. Contact Dermat. 1991;24:86–8.
Miyahara A, Kawashima H, Okubo Y, Hoshika A. A new proposal for a clinical-oriented subclassification of baboon syndrome and a review of baboon syndrome. Asian Pac J Allergy Immunol. 2011;29:150–60.
Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermat. 2004;51:297–310.
Lachapelle J-M, Maibach HI. Patch testing and prick testing: a practical guide official publication of the ICDRG. 4th ed. Cham: Springer; 2020.
White CR. Histopathology of exogenous and systemic contact eczema. Semin Dermatol. 1990;9:226–9.
Özkaya E, Yazganoğlu KD. General aspects of adverse cutaneous drug reactions. In: Özkaya E, Yazganoğlu KD, editors. Adverse cutaneous drug reactions to cardiovascular drugs. London: Springer; 2014. p. 3–63.
Jensen CS, Lisby S, Larsen JK, Veien NK, Menné T. Characterization of lymphocyte subpopulations and cytokine profiles in peripheral blood of nickel-sensitive individuals with systemic contact dermatitis after oral nickel exposure. Contact Dermat. 2004;50:31–8.
Michel M, Dompmartin A, Szczurko C, Casttel B, Moreau A, Leroy D. Eczematous-like drug eruption induced by synergistins. Contact Dermat. 1996;34:86–7.
Goossens C, Sass U, Song M. Baboon syndrome. Dermatology. 1997;194:421–2.
Ash S, Scheman AJ. Systemic contact dermatitis to hydroxyzine. Am J Contact Dermat. 1997;8:2–5.
Reid N, Jariwala S, Hudes G, Fodeman J, Jerschow E, Rosenstreich D. Worsening of contact dermatitis by oral hydroxyzine: a case report. Dermatol Online J. 2013;19:4.
Browne F, Wilkinson SM. Effective prescribing in steroid allergy: controversies and cross-reactions. Clin Dermatol. 2011;29:287–94.
Bircher AJ, Bigliardi P, Zaugg T, Mäkinen-Kiljunen S. Delayed generalized allergic reactions to corticosteroids. Dermatol Basel Switz. 2000;200:349–51.
Lauerma AI, Reitamo S, Maibach HI. Systemic hydrocortisone/cortisol induces allergic skin reactions in presensitized subjects. J Am Acad Dermatol. 1991;24:182–5.
Baeck M, Chemelle JA, Goossens A, Nicolas JF, Terreux R. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367–74.
Hindson C. Contact eczema from methyl salicylate reproduced by oral aspirin (acetyl salicylic acid). Contact Dermat. 1977;3:348–9.
Sánchez-Borges M, Capriles-Hulett A, Caballero-Fonseca F. Risk of skin reactions when using ibuprofen-based medicines. Expert Opin Drug Saf. 2005;4:837–48.
Lakshmi C, Srinivas CR. Systemic (allergic) contact dermatitis to diclofenac. Indian J Dermatol Venereol Leprol. 2011;77:536.
El Sayed F, Bayle-Lebey P, Marguery MC, Bazex J. Systemic sensitization to 17-beta estradiol induced by transcutaneous administration. Ann Dermatol Venereol. 1996;123:26–8.
Jacob SE, Zapolanski T, Chayavichitsilp P. Sensitivity to para-phenylenediamine and intolerance to hydrochlorothiazide. Dermatitis. 2008;19:E44–5.
Malinauskiene L, Isaksson M, Bruze M. Systemic contact dermatitis in a gold-allergic patient after treatment with an oral homeopathic drug. J Am Acad Dermatol. 2013;68:e58.
Glatz M, Hofbauer GFL. Phototoxic and photoallergic cutaneous drug reactions. In: French LE, editor. Adverse cutaneous drug eruptions. Chem Immunol Allergy. Basel: Karger; 2012. p. 167–79.
Stein KR, Scheinfeld NS. Drug-induced photoallergic and phototoxic reactions. Expert Opin Drug Saf. 2007;6:431–43.
Gould JW, Mercurio MG, Elmets CA. Cutaneous photosensitivity diseases induced by exogenous agents. J Am Acad Dermatol. 1995;33:551–73.
Blakely KM, Drucker AM, Rosen CF. Drug-induced photosensitivity: an update: culprit drugs, prevention and management. Drug Saf. 2019;42:827–47.
Youn JI, Lee HG, Yeo UC, Lee YS. Piroxicam photosensitivity associated with vesicular hand dermatitis. Clin Exp Dermatol. 1993;18:52–4.
Ljunggren B, Hindsén M, Isaksson M. Systemic quinine photosensitivity with photoepicutaneous cross-reactivity to quinidine. Contact Dermat. 1992;26:1–4.
Wolf R, Dorfman B, Krakowski A. Quinidine-induced lichenoid and eczematous photodermatitis. Dermatology. 1987;174:285–9.
Lim HW, Buchness MR, Ashinoff R, Soter NA. Chronic actinic dermatitis: study of the spectrum of chronic photosensitivity in 12 patients. Arch Dermatol. 1990;126:317–23.
Braunstein BL. Dyshidrotic eczema associated with piroxicam photosensitivity. Cutis. 1985;35:485–6.
White IR. Photopatch test in a hydrochlorothiazide drug eruption. Contact Dermat. 1983;9:237–237.
Gómez-Bernal S, Álvarez-Pérez A, Rodríguez-Pazos L, Gutiérrez-González E, Rodríguez-Granados MT, Toribio J. Photosensitivity due to thiazides. Actas Dermosifiliogr. 2012;105:359–66.
Silverberg JI. Adult-onset atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7:28–33.
Brummer GC, Wang LT, Sontheimer RD. A possible role for dupilumab (Dupixent) in the management of idiopathic chronic eczematous eruption of aging. Dermatol Online J. 2018;24:13030/qt55z1f6xh.
Chen JK, Jacob SE, Nedorost ST, Hanifin JM, Simpson EL, Boguniewicz M, et al. A pragmatic approach to patch testing atopic dermatitis patients: clinical recommendations based on expert consensus opinion. Dermatitis. 2016;27:186–92.
Silvestre Salvador JF, Romero-Pérez D, Encabo-Durán B. Atopic dermatitis in adults: a diagnostic challenge. J Investig Allergol Clin Immunol. 2017;27:78–88.
Osawa J, Naito S, Aihara M, Kitamura K, Ikezawa Z, Nakajima H. Evaluation of skin test reactions in patients with non-immediate type drug eruptions. J Dermatol. 1990;17:235–9.
Barbaud A. Drug patch testing in systemic cutaneous drug allergy. Toxicology. 2005;209:209–16.
Barbaud A. Skin testing and patch testing in non-IgE-mediated drug allergy. Curr Allergy Asthma Rep. 2014;14:442.
Wilkinson DS, Fregert S, Magnusson B, Bandmann HJ, Calnan CD, Cronin E, et al. Terminology of contact dermatitis. Acta Derm Venereol. 1970;50:287–92.
Popple A, Williams J, Maxwell G, Gellatly N, Dearman RJ, Kimber I. The lymphocyte transformation test in allergic contact dermatitis: new opportunities. J Immunotoxicol. 2016;13:84–91.
Stejskal VD, Olin RG, Forsbeck M. The lymphocyte transformation test for diagnosis of drug-induced occupational allergy. J Allergy Clin Immunol. 1986;77:411–26.
Spoerri I, Bircher AJ, Link S, Heijnen IAFM, Scherer HK. Delayed-type allergy to cobalt-comparison of a flow cytometric lymphocyte proliferation test with patch testing. Contact Dermat. 2018;79:31–3.
Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32(363–8):ix.
Ferguson J. Photosensitivity due to drugs. Photodermatol Photoimmunol Photomed. 2002;18:262–9.
Boguniewicz M, Fonacier L, Guttman-Yassky E, Ong PY, Silverberg J, Farrar JR. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120(10–22):e2.
Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785–92.
Acknowledgements
We acknowledge Carol Mita, MLIS, for her assistance with our literature search.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflict of interest
Amy E. Blum and Susan Burgin have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
Both authors made substantial contributions to the article’s conception and design. AEB performed the literature search and wrote the initial draft. SB provided expert guidance. Both authors were involved in revisions. Both provided final approval of the manuscript and agree to be accountable for all aspects of the work.
Rights and permissions
About this article
Cite this article
Blum, A.E., Burgin, S. Eczematous Drug Eruptions. Am J Clin Dermatol 22, 349–366 (2021). https://doi.org/10.1007/s40257-021-00586-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-021-00586-8