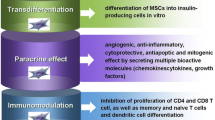
Abstract
Diabetes mellitus is a common lifestyle disease which can be classified into type 1 diabetes mellitus and type 2 diabetes mellitus. While both result in hyperglycemia due to lack of insulin action and further associated chronic ailments, there is a marked distinction in the cause for each type due to which both require a different prophylaxis. As observed, type 1 diabetes is caused due to the autoimmune action of the body resulting in the destruction of pancreatic islet cells. On the other hand, type 2 diabetes is caused either due to insulin resistance of target cells or lack of insulin production as per physiological requirements. Attempts to cure the disease have been made by bringing drastic changes in the patients’ lifestyle; parenteral administration of insulin; prescription of drugs such as biguanides, meglitinides, and amylin; pancreatic transplantation; and immunotherapy. While these attempts cause a certain degree of relief to the patient, none of these can cure diabetes mellitus. However, a new treatment strategy led by the discovery of mesenchymal stem cells and their unique immunomodulatory and multipotent properties has inspired therapies to treat diabetes by essentially reversing the conditions causing the disease. The current review aims to enumerate the role of various mesenchymal stem cells and the different approaches to treat both types of diabetes and its associated diseases as well.





Similar content being viewed by others
References
P. Saeedi et al., “Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition,” Diabetes Res. Clin. Pract., vol. 157, 2019, https://doi.org/10.1016/j.diabres.2019.107843.
Staffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-ll diabetes mellitus (MODY4) linked to IPF1. Nat Genet. 1997;17(2):138–9. https://doi.org/10.1038/ng1097-138.
IDF diabetes atlas, Eighth edition 2017.
T. D. P. Group. Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23(8):857–66. https://doi.org/10.1111/j.1464-5491.2006.01925.x.
Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet (London, England). 2009;373(9680):2027–33. https://doi.org/10.1016/S0140-6736(09)60568-7.
Tiwari P. Recent trends in therapeutic approaches for diabetes management: a comprehensive update. J Diabetes Res. 2015;2015:340838–11. https://doi.org/10.1155/2015/340838.
Marín-Peñalver JJ, Martín-Timón I, Sevillano-Collantes C, Del Cañizo-Gómez FJ. Update on the treatment of type 2 diabetes mellitus. World J Diabetes. Sep. 2016;7(17):354–95. https://doi.org/10.4239/wjd.v7.i17.354.
Otto-Buczkowska E, Jainta N. Pharmacological treatment in diabetes mellitus type 1 - insulin and what else? Int J Endocrinol Metab. Nov. 2017;16(1):–e13008. https://doi.org/10.5812/ijem.13008.
Shomali M. Diabetes treatment in 2025: can scientific advances keep pace with prevalence? Ther Adv Endocrinol Metab. Oct. 2012;3(5):163–73. https://doi.org/10.1177/2042018812465639.
Klingemann H, Matzilevich D, Marchand J. Mesenchymal stem cells - sources and clinical applications. Transfus Med Hemother. Aug. 2008;35(4):272–7. https://doi.org/10.1159/000142333.
Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. May 2011;9:12. https://doi.org/10.1186/1478-811X-9-12.
R. Jiang, “Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes : a pilot study.” Front Med. 2011;5(1):94–100. https://doi.org/10.1007/s11684-011-0116-z.
Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yanez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant. Jun. 2008;14(6):631–40. https://doi.org/10.1016/j.bbmt.2008.01.006.
Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. Mar. 2010;28(3):585–96. https://doi.org/10.1002/stem.269.
Zhou C, Yang B, Tian Y, Jiao H, Zheng W, Wang J, et al. Immunomodulatory effect of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells on lymphocytes. Cell Immunol. 2011;272(1):33–8. https://doi.org/10.1016/j.cellimm.2011.09.010.
Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. May 2006;24(5):1294–301. https://doi.org/10.1634/stemcells.2005-0342.
Anzalone R, Iacono ML, Corrao S, Magno F, Loria T, Cappello F, et al. New emerging potentials for human Wharton’s jelly mesenchymal stem cells: immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev. Dec. 2009;19(4):423–38. https://doi.org/10.1089/scd.2009.0299.
Najar M, Raicevic G, Boufker HI, Kazan HF, Bruyn CD, Meuleman N, et al. Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: combined comparison of adipose tissue, Wharton’s jelly and bone marrow sources. Cell Immunol. 2010;264(2):171–9. https://doi.org/10.1016/j.cellimm.2010.06.006.
Li C, Zhang W, Jiang X, Mao N. Human-placenta-derived mesenchymal stem cells inhibit proliferation and function of allogeneic immune cells. Cell Tissue Res. 2007;330(3):437–46. https://doi.org/10.1007/s00441-007-0504-5.
Yañez R, Lamana ML, García-Castro J, Colmenero I, Ramírez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. Nov. 2006;24(11):2582–91. https://doi.org/10.1634/stemcells.2006-0228.
Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, Eom Y, et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009;259(2):150–6. https://doi.org/10.1016/j.cellimm.2009.06.010.
Shi D, et al. Human adipose tissue−derived mesenchymal stem cells facilitate the immunosuppressive effect of cyclosporin A on T lymphocytes through Jagged-1−mediated inhibition of NF-κB signaling. Exp Hematol. Feb. 2011;39(2):214–224.e1. https://doi.org/10.1016/j.exphem.2010.10.009.
Amable PR, Teixeira MVT, Carias RBV, Granjeiro JM, Borojevic R. Mesenchymal stromal cell proliferation, gene expression and protein production in human platelet-rich plasma-supplemented media. PLoS One. 2014;9(8):e104662. https://doi.org/10.1371/journal.pone.0104662.
Xiong H, Yang XY, Han J, Wang Q, Zou ZL. Cytokine expression patterns and mesenchymal stem cell karyotypes from the bone marrow microenvironment of patients with myelodysplastic syndromes. Brazilian J Med Biol Res = Rev Bras Pesqui medicas e Biol. Jan. 2015;48(3):207–13. https://doi.org/10.1590/1414-431X20144051.
Hwang JH, et al. Comparison of cytokine expression in mesenchymal stem cells from human placenta, cord blood, and bone marrow. J Korean Med Sci. Aug. 2009;24(4):547–54. https://doi.org/10.3346/jkms.2009.24.4.547.
Hsiao ST-F, Asgari A, Lokmic Z, Sinclair R, Dusting GJ, Lim SY, et al. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. Aug. 2012;21(12):2189–203. https://doi.org/10.1089/scd.2011.0674.
Peng L, Jia Z, Yin X, Zhang X, Liu Y, Chen P, et al. Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev. Mar. 2008;17(4):761–74. https://doi.org/10.1089/scd.2007.0217.
van Harmelen V, Röhrig K, Hauner H. Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metab Clin Exp. May 2004;53(5):632–7. https://doi.org/10.1016/j.metabol.2003.11.012.
La Rocca G, et al. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol. Feb. 2009;131(2):267–82. https://doi.org/10.1007/s00418-008-0519-3.
Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. Jan. 2003;21(1):50–60. https://doi.org/10.1634/stemcells.21-1-50.
Heo JS, Choi Y, Kim H-S, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. Jan. 2016;37(1):115–25. https://doi.org/10.3892/ijmm.2015.2413.
Li X, Bai J, Ji X, Li R, Xuan Y, Wang Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med. 2014;34(3):695–704. https://doi.org/10.3892/ijmm.2014.1821.
Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. (Lond). Jul. 2005;2:8. https://doi.org/10.1186/1476-9255-2-8.
Atoui R, Chiu RCJ. Concise review: immunomodulatory properties of mesenchymal stem cells in cellular transplantation: update, controversies, and unknowns. Stem Cells Transl Med. Mar. 2012;1(3):200–5. https://doi.org/10.5966/sctm.2011-0012.
Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. Immunomodulation by mesenchymal stem cells. Diabetes. 2008;57(7):1759 LP–1767. https://doi.org/10.2337/db08-0180.
Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. Nov. 2007;25(11):2739–49. https://doi.org/10.1634/stemcells.2007-0197.
Volarevic V, Arsenijevic N, Lukic ML, Stojkovic M. Concise review: mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells. Jan. 2011;29(1):5–10. https://doi.org/10.1002/stem.556.
Mensah-Brown EPK, Shahin A, Al-Shamisi M, Wei X, Lukic ML. IL-23 leads to diabetes induction after subdiabetogenic treatment with multiple low doses of streptozotocin. Eur J Immunol. Jan. 2006;36(1):216–23. https://doi.org/10.1002/eji.200535325.
Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res. Mar. 2006;98(5):596–605. https://doi.org/10.1161/01.RES.0000207406.94146.c2.
H. Sciences, A. Ain, U. A. Emirates, and S. Medical, “Concise review : mesenchymal stem cell treatment of the complications of diabetes mellitus”. Stem Cells, 2011; pp. 5–10. https://doi.org/10.1002/stem.556.
Subrina J, Ichiro S, Yuichi H, Akira K. Role of angiotensin II in altered expression of molecules responsible for coronary matrix remodeling in insulin-resistant diabetic rats. Arterioscler Thromb Vasc Biol. Nov. 2003;23(11):2021–6. https://doi.org/10.1161/01.ATV.0000094235.78783.D1.
Camp TM, Tyagi SC, Senior RM, Hayden MR, Tyagi SC. Gelatinase B(MMP-9) an apoptotic factor in diabetic transgenic mice. Diabetologia. Oct. 2003;46(10):1438–45. https://doi.org/10.1007/s00125-003-1200-y.
Yoon Y, Uchida S, Masuo O, Cejna M, Park JS, Gwon HC, et al. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circ. Apr. 2005;111(16):2073–85. https://doi.org/10.1161/01.CIR.0000162472.52990.36.
Zhang N, Li J, Luo R, Jiang J, Wang J-A. Bone marrow mesenchymal stem cells induce angiogenesis and attenuate the remodeling of diabetic cardiomyopathy. Exp Clin Endocrinol Diabetes. Feb. 2008;116(2):104–11. https://doi.org/10.1055/s-2007-985154.
Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. Dec. 2004;14(6):1035–41.
Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. Nov. 2006;103(46):17438–43. https://doi.org/10.1073/pnas.0608249103.
Grange C, et al. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci Rep. Mar. 2019;9(1):4468. https://doi.org/10.1038/s41598-019-41100-9.
Kholia S, et al. Mesenchymal stem cell derived extracellular vesicles ameliorate kidney injury in aristolochic acid nephropathy. Front Cell Dev Biol. 2020, [Online]. Available;8:188. https://doi.org/10.3389/fcell.2020.00188.
Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia. Aug. 2000;43(8):957–73. https://doi.org/10.1007/s001250051477.
Kinnaird T, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circ. Mar. 2004;109(12):1543–9. https://doi.org/10.1161/01.CIR.0000124062.31102.57.
Rajashekhar G. Mesenchymal stem cells: new players in retinopathy therapy. Front Endocrinol (Lausanne). 2014;5:59. https://doi.org/10.3389/fendo.2014.00059.
Ritter MR, Banin E, Moreno SK, Aguilar E, Dorrell MI, Friedlander M. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J Clin Invest. Dec. 2006;116(12):3266–76. https://doi.org/10.1172/JCI29683.
Friedlander M, et al. Progenitor cells and retinal angiogenesis. Angiogenesis. Jun. 2007;10(2):89–101. https://doi.org/10.1007/s10456-007-9070-4.
Yang Z, Li K, Yan X, Dong F, Zhao C. Amelioration of diabetic retinopathy by engrafted human adipose-derived mesenchymal stem cells in streptozotocin diabetic rats. Graefes Arch Clin Exp Ophthalmol. Oct. 2010;248(10):1415–22. https://doi.org/10.1007/s00417-010-1384-z.
Elshaer SL, et al. Adipose stem cells and their paracrine factors are therapeutic for early retinal complications of diabetes in the Ins2(Akita) mouse. Stem Cell Res Ther. Nov. 2018;9(1):322. https://doi.org/10.1186/s13287-018-1059-y.
Medina A, Scott PG, Ghahary A, Tredget EE. Pathophysiology of chronic nonhealing wounds. J Burn Care Rehabil. 2005;26(4):306–19.
Spanheimer RG. Correlation between decreased collagen production in diabetic animals and in cells exposed to diabetic serum: response to insulin. Matrix. Apr. 1992;12(2):101–7.
Ariyanti AD, Zhang J, Marcelina O, Nugrahaningrum DA, Wang G, Kasim V, et al. Salidroside-pretreated mesenchymal stem cells enhance diabetic wound healing by promoting paracrine function and survival of mesenchymal stem cells under hyperglycemia. Stem Cells Transl Med. Apr. 2019;8(4):404–14. https://doi.org/10.1002/sctm.18-0143.
Lu H, et al. Salidroside reduces high-glucose-induced podocyte apoptosis and oxidative stress via upregulating heme oxygenase-1 (HO-1) expression. Med Sci Monit. 2017;23:4067–76. https://doi.org/10.12659/msm.902806.
Shi K, Wang X, Zhu J, Cao G, Zhang K, Su Z. Salidroside protects retinal endothelial cells against hydrogen peroxide-induced injury via modulating oxidative status and apoptosis. Biosci Biotechnol Biochem. Sep. 2015;79(9):1406–13. https://doi.org/10.1080/09168451.2015.1038212.
Ariyanti AD, et al. Elevating VEGF-A and PDGF-BB secretion by salidroside enhances neoangiogenesis in diabetic hind-limb ischemia. Oncotarget. 2017;8(57):97187–205. https://doi.org/10.18632/oncotarget.21907.
Zhang J, et al. Inhibition of PHD3 by salidroside promotes neovascularization through cell-cell communications mediated by muscle-secreted angiogenic factors. Sci Rep. Mar. 2017;7:43935. https://doi.org/10.1038/srep43935.
Vojtassak J, et al. Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuro Endocrinol Lett. Dec. 2006;27(Suppl 2):134–7.
Tabatabaei Qomi R, Sheykhhasan M. Adipose-derived stromal cell in regenerative medicine: a review. World J Stem Cells. Aug. 2017;9(8):107–17. https://doi.org/10.4252/wjsc.v9.i8.107.
Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther. Jun. 2017;8(1):145. https://doi.org/10.1186/s13287-017-0598-y.
Moon K-C, et al. Potential of allogeneic adipose-derived stem cell–hydrogel complex for treating diabetic foot ulcers. Diabetes. Apr. 2019;68(4):837 LP–846. https://doi.org/10.2337/db18-0699.
Cao Y, Gang X, Sun C, Wang G. Mesenchymal stem cells improve healing of diabetic foot ulcer. J Diabetes Res. 2017;2017:9328347–10. https://doi.org/10.1155/2017/9328347.
Lopes L, et al. Stem cell therapy for diabetic foot ulcers: a review of preclinical and clinical research. Stem Cell Res Ther. Jul. 2018;9(1):188. https://doi.org/10.1186/s13287-018-0938-6.
Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. Feb. 2007;25(2):371–9. https://doi.org/10.1634/stemcells.2005-0620.
Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, et al. Five-year follow-up after clinical islet transplantation. Diabetes. Jul. 2005;54(7):2060–9.
Yeung TY, et al. Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PLoS ONE. 2012;7(5):1–9. https://doi.org/10.1371/journal.pone.0038189.
Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. May 2005;77(5):587–97. https://doi.org/10.1189/jlb.1104649.
Street CN, Lakey JRT, Shapiro AMJ, Imes S, Rajotte RV, Ryan EA, et al. Islet graft assessment in the Edmonton protocol: implications for predicting long-term clinical outcome. Diabetes. Dec. 2004;53(12):3107–14.
Monaghan M, Helgeson V, Wiebe D. Type 1 diabetes in young adulthood. Curr Diabetes Rev. 2015;11(4):239–50. https://doi.org/10.2174/1573399811666150421114957.
Drexhage HA, Dik WA, Leenen PJM, Versnel MA. The immune pathogenesis of type 1 diabetes: not only thinking outside the cell but also outside the islet and out of the box. Diabetes. Aug. 2016;65(8):2130 LP–2133. https://doi.org/10.2337/dbi16-0030.
Knip M, Siljander H. Autoimmune mechanisms in type 1 diabetes. Autoimmun Rev. 2008;7(7):550–7. https://doi.org/10.1016/j.autrev.2008.04.008.
Yoon JW, Jun HS, Santamaria P. Cellular and molecular mechanisms for the initiation and progression of beta cell destruction resulting from the collaboration between macrophages and T cells. Autoimmunity. 1998;27(2):109–22.
Chhabra P, Brayman KL. Stem cell therapy to cure type 1 diabetes: from hype to hope. Stem Cells Transl Med. May 2013;2(5):328–36. https://doi.org/10.5966/sctm.2012-0116.
Shapiro AM, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. Jul. 2000;343(4):230–8. https://doi.org/10.1056/NEJM200007273430401.
Wu H, Mahato RI. Mesenchymal stem cell-based therapy for type 1 diabetes. Discov Med. Mar. 2014;17(93):139–43.
Dang LT-T, Phan NK, Truong KD. Mesenchymal stem cells for diabetes mellitus treatment: new advances. Biomed Res Ther. 2017;4(1):1062. https://doi.org/10.15419/bmrat.v4i1.144.
Katuchova J, Harvanova D, Spakova T, Kalanin R. Mesenchymal stem cells in the treatment of type 1 diabetes mellitus. Endorc Pathol. 2015;26:95–103. https://doi.org/10.1007/s12022-015-9362-y.
Wang M, Yuan Q, Xie L. Review article mesenchymal stem cell-based immunomodulation : properties and clinical application. Stem Cells Int. 2018;2018:12.
Jiang X-X, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. May 2005;105(10):4120–6. https://doi.org/10.1182/blood-2004-02-0586.
Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. Aug. 2007;25(8):2025–32. https://doi.org/10.1634/stemcells.2006-0548.
Xu G, Zhang Y, Zhang L, Ren G, Shi Y. The role of IL-6 in inhibition of lymphocyte apoptosis by mesenchymal stem cells. Biochem Biophys Res Commun. Sep. 2007;361(3):745–50. https://doi.org/10.1016/j.bbrc.2007.07.052.
Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. Jan. 2008;26(1):151–62. https://doi.org/10.1634/stemcells.2007-0416.
Boumaza I, Srinivasan S, Witt WT, Feghali-Bostwick C, Dai Y, Garcia-Ocana A, et al. Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J Autoimmun. Feb. 2009;32(1):33–42. https://doi.org/10.1016/j.jaut.2008.10.004.
Le Rond S, et al. Evidence to support the role of HLA-G5 in allograft acceptance through induction of immunosuppressive/ regulatory t cells. J Immunol. Mar. 2006;176(5):3266 LP–3276. https://doi.org/10.4049/jimmunol.176.5.3266.
Haddad R, Saldanha-araujo F. Mechanisms of t-cell immunosuppression by mesenchymal stromal cells : what do we know so far ? Biomed Res Int. 2014;2014:14.
Selmani Z, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. Jan. 2008;26(1):212–22. https://doi.org/10.1634/stemcells.2007-0554.
Nasef A, et al. Selected Stro-1-enriched bone marrow stromal cells display a major suppressive effect on lymphocyte proliferation. Int J Lab Hematol. Feb. 2009;31(1):9–19. https://doi.org/10.1111/j.1751-553X.2007.00997.x.
Zhao Y, Lin B, Darflinger R, Zhang Y, Holterman MJ, Skidgel RA. Human cord blood stem cell-modulated regulatory T lymphocytes reverse the autoimmune-caused type 1 diabetes in nonobese diabetic (NOD) mice. PLoS One. 2009;4(1):e4226. https://doi.org/10.1371/journal.pone.0004226.
Volarevic V, Al-Qahtani A, Arsenijevic N, Pajovic S, Lukic ML. Interleukin-1 receptor antagonist (IL-1Ra) and IL-1Ra producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity. Jun. 2010;43(4):255–63. https://doi.org/10.3109/08916930903305641.
Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. Aug. 2007;149(2):353–63. https://doi.org/10.1111/j.1365-2249.2007.03422.x.
Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. Jun. 2004;103(12):4619–21. https://doi.org/10.1182/blood-2003-11-3909.
Maby-El Hajjami H, et al. Functional alteration of the lymphoma stromal cell niche by the cytokine context: role of indoleamine-2,3 dioxygenase. Cancer Res. Apr. 2009;69(7):3228–37. https://doi.org/10.1158/0008-5472.CAN-08-3000.
Ghannam S, Bouffi C, Djouad F, Jorgensen C, Noël D. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res Ther. Mar. 2010;1(1):2. https://doi.org/10.1186/scrt2.
Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. Jan. 2007;109(1):228–34. https://doi.org/10.1182/blood-2006-02-002246.
Ren G, Su J, Zhang L, Zhao X, Ling W, L'huillie A, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. Aug. 2009;27(8):1954–62. https://doi.org/10.1002/stem.118.
Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. Feb. 2005;105(4):1815–22. https://doi.org/10.1182/blood-2004-04-1559.
Nemeth K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. Jan. 2009;15(1):42–9. https://doi.org/10.1038/nm.1905.
Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. Jun. 2009;113(26):6576–83. https://doi.org/10.1182/blood-2009-02-203943.
Quaedackers ME, Baan CC, Weimar W, Hoogduijn MJ. Cell contact interaction between adipose-derived stromal cells and allo-activated T lymphocytes. Eur J Immunol. Dec. 2009;39(12):3436–46. https://doi.org/10.1002/eji.200939584.
De Miguel MP, Pascual CY, Aller MA, Arias J. Immunosuppressive properties of mesenchymal stem cells : advances and applications. Curr Mol Med. 2012;12(5):574–91.
Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. Feb. 2008;2(2):141–50. https://doi.org/10.1016/j.stem.2007.11.014.
Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. Nov. 2007;110(10):3499 LP–3506. https://doi.org/10.1182/blood-2007-02-069716.
Unoki H, Takahashi A, Kawaguchi T, Hara K, Horikoshi M, Andersen G, et al. SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat Genet. Sep. 2008;40(9):1098–102. https://doi.org/10.1038/ng.208.
Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. Feb. 2007;445(7130):881–5. https://doi.org/10.1038/nature05616.
Hani EH, Boutin P, Durand E, Inoue H, Permutt MA, Velho G, et al. Missense mutations in the pancreatic islet beta cell inwardly rectifying K+ channel gene (KIR6.2/BIR): a meta-analysis suggests a role in the polygenic basis of type II diabetes mellitus in Caucasians. Diabetologia. Dec. 1998;41(12):1511–5. https://doi.org/10.1007/s001250051098.
Ali O. Genetics of type 2 diabetes. World J Diabetes. Aug. 2013;4(4):114–23. https://doi.org/10.4239/wjd.v4.i4.114.
Yamauchi T, Tobe K, Tamemoto H, Ueki K, Kaburagi Y, Yamamoto-Honda R, et al. Insulin signalling and insulin actions in the muscles and livers of insulin-resistant, insulin receptor substrate 1-deficient mice. Mol Cell Biol. Jun. 1996;16(6):3074–84. https://doi.org/10.1128/mcb.16.6.3074.
Saini V. Molecular mechanisms of insulin resistance in type 2 diabetes mellitus. World J Diabetes. Jul. 2010;1(3):68–75. https://doi.org/10.4239/wjd.v1.i3.68.
Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. Dec. 2005;115(12):3587–93. https://doi.org/10.1172/JCI25151.
Zang L, Hao H, Liu J, Li Y, Han W, Mu Y. Mesenchymal stem cell therapy in type 2 diabetes mellitus. Diabetol Metab Syndr. May 2017;9:36. https://doi.org/10.1186/s13098-017-0233-1.
Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–84. https://doi.org/10.1002/jcb.20886.
Chen L, Tredget EE, Wu PYG, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. Apr. 2008;3(4):e1886. https://doi.org/10.1371/journal.pone.0001886.
Park CW, et al. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int J stem cells. 2009;2(1):59–68. https://doi.org/10.15283/ijsc.2009.2.1.59.
Bastidas-Coral AP, Bakker AD, Zandieh-Doulabi B, Kleverlaan CJ, Bravenboer N, Forouzanfar T, et al. Cytokines TNF-α, IL-6, IL-17F, and IL-4 differentially affect osteogenic differentiation of human adipose stem cells. Stem Cells Int. 2016;2016:1318256–9. https://doi.org/10.1155/2016/1318256.
Cuerquis J, Romieu-Mourez R, François M, Routy JP, Young YK, Zhao J, et al. Human mesenchymal stromal cells transiently increase cytokine production by activated T cells before suppressing T-cell proliferation: effect of interferon-γ and tumor necrosis factor-α stimulation. Cytotherapy. Feb. 2014;16(2):191–202. https://doi.org/10.1016/j.jcyt.2013.11.008.
Skyler JS, Fonseca VA, Segal KR, Rosenstock J, Investigators M-D. Allogeneic mesenchymal precursor cells in type 2 diabetes: a randomized, placebo-controlled, dose-escalation safety and tolerability pilot study. Diabetes Care. Sep. 2015;38(9):1742–9. https://doi.org/10.2337/dc14-2830.
Kong D, et al. Umbilical cord mesenchymal stem cell transfusion ameliorated hyperglycemia in patients with type 2 diabetes mellitus. Clin Lab. 2014;60(12):1969–76.
Liu X, et al. A preliminary evaluation of efficacy and safety of Wharton’s jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cell Res Ther. Apr. 2014;5(2):57. https://doi.org/10.1186/scrt446.
Miao X-Y, Gu ZY, Liu P, Hu Y, Li L, Gong YP, et al. The human glucagon-like peptide-1 analogue liraglutide regulates pancreatic beta-cell proliferation and apoptosis via an AMPK/mTOR/P70S6K signaling pathway. Peptides. 2013;39:71–9. https://doi.org/10.1016/j.peptides.2012.10.006.
Shao S, Nie M, Chen C, Chen X, Zhang M, Yuan G, et al. Protective action of liraglutide in beta cells under lipotoxic stress via PI3K/Akt/FoxO1 pathway. J Cell Biochem. Jun. 2014;115(6):1166–75. https://doi.org/10.1002/jcb.24763.
W. Wang et al., “Liraglutide combined with human umbilical cord mesenchymal stem cell transplantation inhibits beta-cell apoptosis via mediating the ASK1/JNK/BAX pathway in rats with type 2 diabetes,” Diabetes Metab Res Rev, vol. 0, no. 0, p. e3212, 2019, https://doi.org/10.1002/dmrr.3212.
Deng Z, Xu H, Zhang J, Yang C, Jin L, Liu J, et al. Infusion of adipose-derived mesenchymal stem cells inhibits skeletal muscle Mitsugumin 53 elevation and thereby alleviates insulin resistance in type 2 diabetic rats. Mol Med Rep. 2018;17(6):8466–74. https://doi.org/10.3892/mmr.2018.8901.
Si Y, Zhao Y, Hao H, Liu J, Guo Y, Mu Y, et al. Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: identification of a novel role in improving insulin sensitivity. Diabetes. Jun. 2012;61(6):1616–25. https://doi.org/10.2337/db11-1141.
Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. https://doi.org/10.1146/annurev-physiol-021909-135846.
Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. Nov. 2009;58(11):2574–82. https://doi.org/10.2337/db08-1475.
Zhang Q-Z, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. Oct. 2010;28(10):1856–68. https://doi.org/10.1002/stem.503.
Geng Y, et al. Mesenchymal stem cells ameliorate rhabdomyolysis-induced acute kidney injury via the activation of M2 macrophages. Stem Cell Res Ther. Jun. 2014;5(3):80. https://doi.org/10.1186/scrt469.
Guney MA, Gannon M. Pancreas cell fate. Birth Defects Res C Embryo Today. Sep. 2009;87(3):232–48. https://doi.org/10.1002/bdrc.20156.
Harrison KA, Thaler J, Pfaff SL, Gu H, Kehrl JH. Pancreas dorsal lobe agenesis and abnormal islets of Langerhans in Hlxb9 -deficient mice. Nat Genet. 1999;23:71–75.
Kawaguchi Y, Cooper B, Gannon M, Ray M, Macdonald RJ, Wright CVE. The role of the transcriptional regulator Ptf1a in converting intestinal to. Nat Genet. 2002;32:128–134. https://doi.org/10.1038/ng959.
Li H, Arber S, Jessell TM, Edlund H. Selective agenesis of the dorsal pancreas in mice lacking homeobox gene Hlxb9. Nat Genet. 1999;23:67–70.
Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122(3):983–95.
Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. β-cell-specific inactivation of the mouseIpf1/Pdx1 gene results in loss of the β-cell phenotype and maturity onset diabetes. Genes Dev. Jun. 1998;12(12):1763–8. https://doi.org/10.1101/gad.12.12.1763.
Gannon M, Tweedie Ables E, Crawford L, Lowe D, Offield MF, Magnuson MA, et al. pdx-1 function is specifically required in embryonic beta cells to generate appropriate numbers of endocrine cell types and maintain glucose homeostasis. Dev Biol. Feb. 2008;314(2):406–17. https://doi.org/10.1016/j.ydbio.2007.10.038.
Dutta S, Bonner-weir S. Inhibition of ICE slows ALS in mice. Nature. 1998;392:8–10.
Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet. Oct. 1997;17(2):138–9. https://doi.org/10.1038/ng1097-138.
Stoffers DA, Stanojevic V, Habener JF. Insulin promoter factor-1 gene mutation linked to early-onset type 2 diabetes mellitus directs expression of a dominant negative isoprotein. J Clin Investigvol. 1998;1.
Chakrabarti SK, James JC, Mirmira RG. Quantitative assessment of gene targeting in vitro and in vivo by the pancreatic transcription factor, Pdx1: importance of chromatin structure in directing promoter binding. J Biol Chem. Apr. 2002;277(15):13286–93. https://doi.org/10.1074/jbc.M111857200.
Martin CC, Oeser JK, O’Brien RM. Differential regulation of islet-specific glucose-6-phosphatase catalytic subunit-related protein gene transcription by Pax-6 and Pdx-1. J Biol Chem. Aug. 2004;279(33):34277–89. https://doi.org/10.1074/jbc.M404830200.
Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12(11):4251–9.
Raum JC, Gerrish K, Artner I, Henderson E, Guo M, Sussel L, et al. FoxA2, Nkx2.2, and PDX-1 regulate islet beta-cell-specific mafA expression through conserved sequences located between base pairs -8118 and -7750 upstream from the transcription start site. Mol Cell Biol. Aug. 2006;26(15):5735–43. https://doi.org/10.1128/MCB.00249-06.
Smith SB, Watada H, Scheel DW, Mrejen C, German MS. Autoregulation and maturity onset diabetes of the young transcription factors control the human PAX4 promoter. J Biol Chem. 2000;275(47):36910–9. https://doi.org/10.1074/jbc.M005202200.
Ohneda K, Mirmira RG, Wang J, Johnson JD, German MS. The homeodomain of pdx-1 mediates multiple protein-protein interactions in the formation of a transcriptional activation complex on the insulin promoter. Mol Cell Biol. Feb. 2000;20(3):900 LP–911. https://doi.org/10.1128/MCB.20.3.900-911.2000.
Peers B, Leonard J, Sharma S, Teitelman G, Montminy MR. Insulin expression in pancreatic islet cells relies on cooperative interactions between the helix loop helix factor E47 and the homeobox factor STF-1. Mol Endocrinol. Dec. 1994;8(12):1798–806. https://doi.org/10.1210/mend.8.12.7708065.
Lottmann H, Vanselow J, Hessabi B, Walther R. The Tet-On system in transgenic mice: inhibition of the mouse pdx-1 gene activity by antisense RNA expression in pancreatic beta-cells. J Mol Med (Berl). Jun. 2001;79(5–6):321–8.
Waeber G, Thompson N, Nicod P, Bonny C. Transcriptional activation of the GLUT2 gene by the IPF-1/STF-1/IDX-1 homeobox factor. Mol Endocrinol. Nov. 1996;10(11):1327–34. https://doi.org/10.1210/mend.10.11.8923459.
Wang H, Maechler P, Ritz-Laser B, Hagenfeldt KA, Ishihara H, Philippe J, et al. Pdx1 level defines pancreatic gene expression pattern and cell lineage differentiation. J Biol Chem. Jul. 2001;276(27):25279–86. https://doi.org/10.1074/jbc.M101233200.
Macfarlane WM, Campbell SC, Elrick LJ, Oates V, Bermano G, Lindley KJ, et al. Glucose regulates islet amyloid polypeptide gene transcription in a PDX1- and calcium-dependent manner. J Biol Chem. May 2000;275(20):15330–5. https://doi.org/10.1074/jbc.M908045199.
Watada H, Kajimoto Y, Kaneto H, Matsuoka TA, Fujitani Y, Miyazaki JI, et al. Involvement of the homeodomain-containing transcription factor PDX-1 in islet amyloid polypeptide gene transcription. Biochem Biophys Res Commun. Dec. 1996;229(3):746–51.
Watada H, Kajimoto Y, Miyagawa JI, Hanafusa T, Hamaguchi K, Matsuoka TA, et al. PDX-1 induces insulin and glucokinase gene expressions in αTC1 clone 6 cells in the presence of betacellulin. Diabetes. 1996;45(12):1826 LP–1831. https://doi.org/10.2337/diab.45.12.1826.
Watada H, et al. The human glucokinase gene β-cell-type promoter: an essential role of insulin promoter factor 1/PDX-1 in its activation in HIT-T15 cells. Diabetes. Nov. 1996;45(11):1478 LP–1488. https://doi.org/10.2337/diab.45.11.1478.
Krapp A, et al. The bHLH protein PTF1-p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev. Dec. 1998;12(23):3752–63.
Burlison JS, Long Q, Fujitani Y, Wright CVE, Magnuson MA. Pdx-1 and Ptf1a concurrently determine fate specification of pancreatic multipotent progenitor cells. Dev Biol. Apr. 2008;316(1):74–86. https://doi.org/10.1016/j.ydbio.2008.01.011.
Wiebe PO, et al. Ptf1a binds to and activates area III, a highly conserved region of the Pdx1 promoter that mediates early pancreas-wide Pdx1 expression. Mol Cell Biol. Jun. 2007;27(11):4093–104. https://doi.org/10.1128/MCB.01978-06.
Apelqvist A, Ahlgren U, Edlund H. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr Biol. Oct. 1997;7(10):801–4.
Villasenor A, Chong DC, Cleaver O. Biphasic Ngn3 expression in the developing pancreas. Dev Dyn. Nov. 2008;237(11):3270–9. https://doi.org/10.1002/dvdy.21740.
Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. Feb. 2000;97(4):1607–11.
Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. May 2002;129(10):2447–57.
Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. Aug. 2009;138(3):449–62. https://doi.org/10.1016/j.cell.2009.05.035.
Jacquemin P, Durviaux SM, Jensen J, Godfraind C, Gradwohl G, Guillemot F̧, et al. Transcription factor hepatocyte nuclear factor 6 regulates pancreatic endocrine cell differentiation and controls expression of the proendocrine gene ngn3. Mol Cell Biol. Jun. 2000;20(12):4445–54.
Jacquemin P, Lemaigre FP, Rousseau GG. The Onecut transcription factor HNF-6 (OC-1) is required for timely specification of the pancreas and acts upstream of Pdx-1 in the specification cascade. Dev Biol. Jun. 2003;258(1):105–16.
Gannon M, Ray MK, Van Zee K, Rausa F, Costa RH, Wright CV. Persistent expression of HNF6 in islet endocrine cells causes disrupted islet architecture and loss of beta cell function. Development. Jul. 2000;127(13):2883–95.
Tweedie E, Artner I, Crawford L, Poffenberger G, Thorens B, Stein R, et al. Maintenance of hepatic nuclear factor 6 in postnatal islets impairs terminal differentiation and function of beta-cells. Diabetes. Dec. 2006;55(12):3264–70. https://doi.org/10.2337/db06-0090.
Burke Z, Oliver G. Prox1 is an early specific marker for the developing liver and pancreas in the mammalian foregut endoderm. Mech Dev. Oct. 2002;118(1–2):147–55.
Matsuoka T, Artner I, Henderson E, Means A, Sander M, Stein R. The MafA transcription factor appears to be responsible for tissue-specific expression of insulin. Proc Natl Acad Sci U S A. Mar. 2004;101(9):2930–3. https://doi.org/10.1073/pnas.0306233101.
Olbrot M, Rud J, Moss LG, Sharma A. Identification of beta-cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA. Proc Natl Acad Sci U S A. May 2002;99(10):6737–42. https://doi.org/10.1073/pnas.102168499.
Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. Jun. 2005;25(12):4969–76. https://doi.org/10.1128/MCB.25.12.4969-4976.2005.
Chen Y, Pan FC, Brandes N, Afelik S, Solter M, Pieler T. Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Dev Biol. Jul. 2004;271(1):144–60. https://doi.org/10.1016/j.ydbio.2004.03.030.
Ostrom M, et al. Retinoic acid promotes the generation of pancreatic endocrine progenitor cells and their further differentiation into beta-cells. PLoS One. Jul. 2008;3(7):e2841. https://doi.org/10.1371/journal.pone.0002841.
Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. Mar. 1997;386(6623):399–402. https://doi.org/10.1038/386399a0.
Wang J, Elghazi L, Parker SE, Kizilocak H, Asano M, Sussel L, et al. The concerted activities of Pax4 and Nkx2.2 are essential to initiate pancreatic beta-cell differentiation. Dev Biol. Feb. 2004;266(1):178–89.
Wang Q, Elghazi L, Martin S, Martins I, Srinivasan RS, Geng X, et al. Ghrelin is a novel target of Pax4 in endocrine progenitors of the pancreas and duodenum. Dev Dyn. Jan. 2008;237(1):51–61. https://doi.org/10.1002/dvdy.21379.
Doyle MJ, Loomis ZL, Sussel L. Nkx2.2-repressor activity is sufficient to specify alpha-cells and a small number of beta-cells in the pancreatic islet. Development. Feb. 2007;134(3):515–23. https://doi.org/10.1242/dev.02763.
Sussel L, Kalamaras J, Hartigan-O'Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, et al. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. Jun. 1998;125(12):2213–21.
Rorsman P, Salehi SA, Abdulkader F, Braun M, MacDonald PE. K(ATP)-channels and glucose-regulated glucagon secretion. Trends Endocrinol Metab. Oct. 2008;19(8):277–84. https://doi.org/10.1016/j.tem.2008.07.003.
Heimberg H, De Vos A, Pipeleers D, Thorens B, Schuit F. Differences in glucose transporter gene expression between rat pancreatic alpha- and beta-cells are correlated to differences in glucose transport but not in glucose utilization. J Biol Chem. Apr. 1995;270(15):8971–5.
Heimberg H, et al. The glucose sensor protein glucokinase is expressed in glucagon-producing alpha-cells. Proc Natl Acad Sci U S A. Jul. 1996;93(14):7036–41. https://doi.org/10.1073/pnas.93.14.7036.
Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. Apr. 2010;464(7292):1149–54. https://doi.org/10.1038/nature08894.
Urbán VS, et al. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. Jan. 2008;26(1):244–53. https://doi.org/10.1634/stemcells.2007-0267.
Cai J, et al. Umbilical cord mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: a pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. Diabetes Care. Jan. 2016;39(1):149 LP–157. https://doi.org/10.2337/dc15-0171.
D’Addio F, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in new-onset type 1 diabetes: a multicenter analysis. Diabetes. Sep. 2014;63(9):3041 LP–3046. https://doi.org/10.2337/db14-0295.
Carlsson P-O, Schwarcz E, Korsgren O, Le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. Feb. 2015;64(2):587 LP–592. https://doi.org/10.2337/db14-0656.
Ito K, et al. A novel method to isolate mesenchymal stem cells from bone marrow in a closed system using a device made by nonwoven fabric. Tissue Eng Part C Methods. Apr. 2009;16(1):81–91. https://doi.org/10.1089/ten.tec.2008.0693.
Otsuru S, Hofmann TJ, Olson TS, Dominici M, Horwitz EM. Improved isolation and expansion of bone marrow mesenchymal stromal cells using a novel marrow filter device. Cytotherapy. 2013;15(2):146–53. https://doi.org/10.1016/j.jcyt.2012.10.012.
Madeira C, Santhagunam A, Cabral JMS. Advanced cell therapies for articular cartilage regenerationf. Trends Biotechnol. 2014. pp 1–8. https://doi.org/10.1016/j.tibtech.2014.11.003.
Gattás-Asfura KM, Stabler CL. Bioorthogonal layer-by-layer encapsulation of pancreatic islets via hyperbranched polymers. ACS Appl Mater Interfaces. Oct. 2013;5(20):9964–74. https://doi.org/10.1021/am401981g.
Tomei AA, Manzoli V, Fraker CA, Giraldo J, Velluto D, Najjar M. Device design and materials optimization of conformal coating for islets of Langerhans. PNAS. 2014;111(29). https://doi.org/10.1073/pnas.1402216111.
Mravic M, Péault B, James AW. Current trends in bone tissue engineering. Biomed Res Int. 2014;2014:1–5. https://doi.org/10.1155/2014/865270.
Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cells Dev. 2015;27:1–43.
P. Kong, X. Xie, F. Li, Y. Liu, and Y. Lu, “Biochemical and biophysical research communications placenta mesenchymal stem cell accelerates wound healing by enhancing angiogenesis in diabetic Goto-Kakizaki ( GK ) rats,” Biochem Biophys Res Commun, no. 2013, https://doi.org/10.1016/j.bbrc.2013.07.088.
Ledesma-martínez E, Mendoza-núñez VM, Santiago-osorio E. Mesenchymal stem cells derived from dental pulp : a review. Stem Cells Dev. 2016;2016.
Fuller B. Cryoprotectants: the essential antifreezes to protect life in the frozen state. Cryo Letters. 2004;25.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Goenka, V., Borkar, T., Desai, A. et al. Therapeutic potential of mesenchymal stem cells in treating both types of diabetes mellitus and associated diseases. J Diabetes Metab Disord 19, 1979–1993 (2020). https://doi.org/10.1007/s40200-020-00647-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-020-00647-5