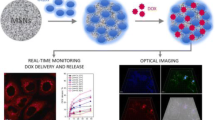
Abstract
Background and the purpose of the study
The purpose of the present research was to synthesize affordable nanoparticles for simultaneous drug release and cell fluorescence imaging to decrease the costs associated with conventional treatments.
Methods
In the present study, N-doped carbon nanodots@UiO-66-NH2 nanoparticles were simply synthesized in few steps and were used as a novel carrier for rosmarinic acid (RA). Nano particles were characterized by FT-IR spectroscopy, X-ray powder diffraction (XRD), Dynamic Light Scattering (DLS) and field emission scanning electron microscopy (FE-SEM). UV/vis spectroscopy was used to study the release profile of RA drug from this novel carrier. Methylthiazolyl tetrazolium (MTT) assay was to evaluate the effect of irradiation with a (UV) lamp. Confocal laser scanning microscopy was used for fluorescence imaging of cancer cells.
Results
Results of the MTT assay revealed that UiO-66-NH2@N-CNDs nanoparticles as a drug carrier for RA, have an excellent therapeutic effect due to their high quantum yield under irradiation of UV light. On the contrary, the observed therapeutic effect was decreased under ambient light.
Conclusions
UiO-66-NH2@N-CNDs nanoparticles can be considered as promising vehicles for drug delivery due to their cost effectiveness in cancer treatment, based on the results of MTT assay. It should be emphasized that this nanocarrier can be as potential platforms for coincident drug delivery system and cell fluorescence imaging due to possessing green fluorescence and microporosity features.

Graphical abstract










Similar content being viewed by others
References
Harborne JB. Caffeic acid ester distribution in higher plants. Z Naturforsch. 1966;21b:604–5.
Litvinenko VI, Popova TP, Simonjon AV, Zoz IG, Sokolov VS. Gerbstoffe und oxyzimstaureabkommlinge in Labiaten. Planta Med. 1975;27:372–80.
Yang R, Shetty K. Stimulation of rosmarinic acid in shoot cultures of oregano (Origanum vulgare) clonal line in response to proline, proline analogue, and proline precursors. J Agr Food Chem. 1998;46(7):2888–93.
Al-Sereiti MR, Abu-Amer KM, Sen P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian J Exp Biol. 1999;37:124–30.
Inatani R, Nakatani N, Fuwa H. Antioxidative effect of constituents of rosemary (Rosmarinus officinalis L.) and their derivatives. Agr Biol Chem. 1983;47:521–8.
Zhu BT, Loder DP, Cai MX, Ho CT, Huang MT, Conney AH. Dietary administration of an extract from rosemary leaves enhances the liver microsomal metabolism of endogenous estrogens and decreases their uterotropic action in CD-1 mice. Carcinogenesis. 1998;19(10):1821–7.
Kim HK, Lee HK, Shin CG, Huh H. HIV: integrase inhibitory activity of agastache rugosa. Arch Pharm Research. 1999;22(5):520–3.
Makino T, Ono T, Muso E, Yoshida H, Honda G, Sasayama S. Inhibitory effects of rosmarinic acid on the proliferation of cultured murine mesangial cells. Nephrol Dial Transplant. 2000;15:1140–5.
Kelm MA, Nair MG, Strasburg GM, De Witt DL. Antioxidant and cyclooxygenase inhibitory phenolic compounds from ocimum sanctum Linn. Phytomedicine. 2000;7(1):7–13.
Casanova F, Estevinho BN, Santos L. Preliminary studies of rosmarinic acid microencapsulation with chitosan and modified chitosan for topical delivery. Powder Technol. 2016;297:44–9.
Davis ME. Ordered porous materials for emerging applications. Nature. 2002;417(6891):813–21.
Li JR, Sculley J, Zhou HC. Metal–organic frameworks for separations. Chem Rev. 2011;112(2):869–932.
Lee J, Farha OK, Roberts J, Scheidt KA, Nguyen ST, Hupp JT. Metal–organic framework materials as catalysts. Chem Soc Rev. 2009;38(5):1450–9.
Ma S, Zhou HC. Gas storage in porous metal–organic frameworks for clean energy applications. Chem Comm. 2010;46(1):44–53.
Mason JA, Veenstra M, Long JR. Evaluating metal–organic frameworks for natural gas storage. Chem Sci. 2014;5(1):32–51.
Evans OR, Lin W. Crystal engineering of NLO materials based on metal−organic coordination networks. Account Chem Res. 2002;35(7):511–22.
Kent CA, Mehl BP, Ma L, Papanikolas JM, Meyer TJ, Lin W. Energy transfer dynamics in metal−organic frameworks. J Am Chem Soc. 2010;132(37):12767–9.
Liu D, Lu K, Poon C, Lin W. Metal–organic frameworks as sensory materials and imaging agents. Inorg Chem. 2013;53(4):1916–24.
Horcajada P, Gref R, Baati T, Allan PK, Maurin G, Couvreur P, et al. Metal–organic frameworks in biomedicine. Chem Rev. 2011;112(2):1232–68.
Baati T, Njim L, Neffati F, Kerkeni A, Bouttemi M, Gref R, et al. In depth analysis of the in vivo toxicity of nanoparticles of porous iron (III) metal–organic frameworks. Chem Sci. 2013;4(4):1597–607.
Della Rocca J, Liu D, Lin W. Nanoscale metal–organic frameworks for biomedical imaging and drug delivery. Account Chem Res. 2011;44(10):957–68.
Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T, et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater. 2010;9(2):172–8.
Sun CY, Qin C, Wang CG, Su ZM, Wang S, Wang XL, et al. Chiral nanoporous metal-organic frameworks with high porosity as materials for drug delivery. Adv Mater. 2011;23(47):5629–32.
He C, Lu K, Liu D, Lin W. Nanoscale metal–organic frameworks for the co-delivery of cisplatin and pooled siRNAs to enhance therapeutic efficacy in drug-resistant ovarian cancer cells. J Am Chem Soc. 2014;136(14):5181–4.
Zhuang J, Kuo CH, Chou LY, Liu DY, Weerapana E, Tsung CK. Optimized metal–organic-framework nanospheres for drug delivery: evaluation of small-molecule encapsulation. ACS Nano. 2014;8(3):2812–9.
Lin W, Hu Q, Jiang K, Cui Y, Yang Y, Qian G. A porous Zn-based metal-organic framework for pH and temperature dual-responsive controlled drug release. Microporous Mesoporous Mat. 2017;249:55–60.
Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–82.
Dyson PJ, Sava G. Metal-based antitumour drugs in the post genomic era. Dalton Trans. 2006;(16):1929–33.
Manoochehri S, Darvishi B, Kamalinia G, Amini M, Fallah M, Ostad SN, et al. Surface modification of PLGA nanoparticles via human serum albumin conjugation for controlled delivery of docetaxel. DARU. 2013;21(1):58.
Vallet-Regi M, Ramila A, Del Real RP, Pérez-Pariente J. A new property of MCM-41: drug delivery system. Chem Mater. 2001;13(2):308–11.
Cai W, Chu CC, Liu G, Wáng YXJ. Metal–organic framework-based nanomedicine platforms for drug delivery and molecular imaging. Small. 2015;11(37):4806–22.
Anand R, Borghi F, Manoli F, Manet I, Agostoni V, Reschiglian P, et al. Host–guest interactions in Fe (III)-trimesate MOF nanoparticles loaded with doxorubicin. J Phys Chem B. 2014;118(29):8532–9.
He L, Wang T, An J, Li X, Zhang L, Li L, et al. Carbon nanodots@zeolitic imidazolate framework-8 nanoparticles for simultaneous pH-responsive drug delivery and fluorescence imaging. CrystEngComm. 2014;16(16):3259–63.
Wang L, Zhu SJ, Wang HY, Qu SN, Zhang YL, Zhang JH, et al. Common origin of green luminescence in carbon nanodots and graphene quantum dots. ACS Nano. 2014;8(3):2541–7.
Tetsuka H, Asahi R, Nagoya A, Okamoto K, Tajima I, Ohta R, et al. Optically tunable amino-functionalized graphene quantum dots. Adv Mater. 2012;24(39):5333–8.
Martindale BC, Hutton GA, Caputo CA, Prantl S, Godin R, Durrant JR, et al. Enhancing light absorption and charge transfer efficiency in carbon dots through graphitization and core nitrogen doping. Angew Chem Int Ed. 2017;56(23):6459–63.
Xiao Q, Liang Y, Zhu F, Lu S, Huang S. Microwave-assisted one-pot synthesis of highly luminescent N-doped carbon dots for cellular imaging and multi-ion probing. Microchim Acta. 2017;184(7):2429–38.
Sun YP, Zhou B, Lin Y, Wang W, Fernando KS, Pathak P, et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc. 2006;128(24):7756–7.
Ruyra A, Yazdi A, Espín J, Carné-Sánchez A, Roher N, Lorenzo J, et al. Synthesis, culture medium stability, and in vitro and in vivo zebrafish embryo toxicity of metal–organic framework nanoparticles. Chem Eur J. 2015;21(6):2508–18.
Lu S, Guo S, Xu P, Li X, Zhao Y, Gu W, et al. Hydrothermal synthesis of nitrogen-doped carbon dots with real-time live-cell imaging and blood–brain barrier penetration capabilities. Int J Nanomedicine. 2016;11:6325–36.
Haydar MA, Abid HR, Sunderland B, Wang S. Metal organic frameworks as a drug delivery system for flurbiprofen. Drug Des Dev Ther. 2017;11:2685–95.
Acknowledgements
We gratefully acknowledge to the Research Council University of Guilan for the partial support of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 298 kb)
Rights and permissions
About this article
Cite this article
Tabatabaeian, K., Simayee, M., Fallah-Shojaie, A. et al. N-doped carbon nanodots@UiO-66-NH2 as novel nanoparticles for releasing of the bioactive drug, rosmarinic acid and fluorescence imaging. DARU J Pharm Sci 27, 307–315 (2019). https://doi.org/10.1007/s40199-019-00276-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-019-00276-1