Abstract
In order to leverage the concept of integrated computational materials engineering (ICME) for manufacturing of high-performance automotive transmission components such as steel gears, it is important to bring a closer collaboration between geometrical design, material selection, and manufacturing design stages for these components. This can be achieved by making the manufacturers aware of the implications of the decisions taken during each of these stages on material and its underlying microstructure. In order to facilitate this, it is necessary to model the evolution of the microstructure in the process-chain and its resultant properties. With this view, the current work focusses on development of an integrated modeling scheme of carburizing, quenching, and tempering processes using chemical composition-dependent, microstructure-based models intended to be used in an ICME framework for steel gear manufacturing. The individual process models are implemented in the commercial FEM suite ABAQUS™, with essential microstructure physics incorporated via user-subroutines. The individual process-models and their sequential integration are validated against experimental case-studies from literature. After validation, the integrated modeling scheme is automated by writing appropriate pre-processing and post-processing wrapper scripts, leading to the development of an independent manufacturing module that can be used in an ICME workflow. Finally, the utility of this module is demonstrated by using it for the exploration of the manufacturing process design and material selection scenarios for the production of a typical spur gear.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing the service life of components such as automotive gears, or enhancing their performance, is always an aspect of interest and concern for the industry. This requires careful synthesis of the geometrical design of the component, material selection and its manufacturing process with a close interaction among the three stages. In the traditional design and development approach, however, consideration of these interactions is weak. For example, in the traditional approach, the decisions during the geometric designing of the component as well as during process design are taken based on limited descriptors of material and its underlying microstructure. Because of this, there is always a tendency of over-designing the component and processes in order to achieve the desired performance. This limitation leads to a higher production cost. An integrated approach of product development which enables tighter coupling between geometrical design, material selection, and manufacturing through detailed description of material and its microstructure available at each of these stages is necessary to address this issue. Within the manufacturing process design stage, in order to facilitate the detailed description of the underlying microstructure and its evolution, it is, thus, also important to consider the combined effect of the complete process chain in a through process framework. Integrated computational materials engineering (ICME) is such an approach of designing the products, materials, and manufacturing processes through linking processes and performance material models at various length scales along with the design models by taking advantage of the mathematical modeling and simulation techniques.
A typical gear’s service conditions require a combination of diverse properties in the material such as high surface hardness, wear resistance, impact strength, and toughness. From a material selection and manufacturing point of view, carburizing-quenching-tempering (hereafter referred as C-Q-T) is one of the widely used process chains in industry that imparts these properties to steel. C-Q-T processes involve various metallurgical phenomena occurring at different length scales that result in the desired microstructure of the steel, thereby endowing it with the required properties. Therefore, in order to develop economical high-performance gears, it becomes necessary to model and design the C-Q-T process chain in an integrated fashion along with other design processes.
In the past, many attempts have been made to model C-Q-T processes individually and also in integration to each other for different application scenarios. The majority of these works have made use of empirical or semi-empirical models for microstructure physics in a FDM or FEM modeling framework. Carburizing has been modeled by solving Fick’s laws for carbon diffusion under various process conditions either as 1-D idealization [1,2,3] or taking the exact geometry of the component into account [4, 5]. A large number of studies have also been conducted to model the diffusional phase transformation in steel parts during quenching or cooling from austenitization temperatures using Johnson-Mehl-Avrami-Kolmogorov (JMAK) [6] type equations and relating it to distortion and residual stress generation using 2D and 3D FEM simulations [7,8,9,10]. Conversely, many researchers have also employed other semi-empirical phase transformation models [11, 12] to model phase transformation during cooling. Tempering, apart from precipitation of carbides in a super-saturated martensite, also involves other parallel and overlapping microstructural changes taking place depending upon the temperature of tempering [13]. A number of attempts have been made to model the tempering process specific to the steel composition and to the temperature regime of interest. Most of these attempts involve physically based modeling of tempering either in terms of evolution of carbide precipitates [14, 15] or tempered martensite [16, 17] with time, while others have used indirect methods of modeling tempering in terms of its effect on material properties such as hardness [18,19,20]. Apart from modeling these processes individually, a few attempts have also been made to model these processes in sequence as a process chain for different applications. Most of these attempts have been targeted on the integration of carburizing and quenching simulations in the FEM-based thermo-mechanical modeling framework [21,22,23,24,25] and a relatively few similar studies have been done for the integration of quenching and tempering simulations [15, 26,27,28]. Also, most of these modeling activities cater to a particular grade of steel to which the parameters of the individual models are tuned, thereby failing to give any form of guidance in terms of the chemical composition of the steel required for a specific property. To our knowledge, no attempt has been made to model the combined effect of the C-Q-T processes in integration with each other, in an ICME framework, applicable for a large variety of steels, so as to enable better material and manufacturing process parameter selection for better gear designs. With this view, the present work focusses on the development of thermo-metallurgical through process models of the C-Q-T process chain for an ICME framework. In order to realize this, microstructure-based, chemical composition-dependent models of C-Q-T processes are implemented and integrated on a commercial FEM suite ABAQUSTM with the aid of user-subroutines, to predict final microstructure and hardness distribution. The process models are validated against three validation problems. Finally, an automated scheme is established to integrate these process models in a sequential order, leading to the development of a stand-alone module that takes gear geometry, chemical composition, and process parameters as inputs and gives key material and design outputs. The module is intended to be used in establishing an ICME workflow for automotive gears. In order to demonstrate the application of such a module, case studies for manufacturing process design and material selection for a typical automotive gear are carried out.
Mathematical Modeling of the Process Chain
Modeling Microstructure and Property Evolution
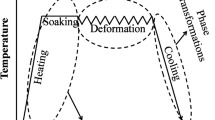
Figure 1 describes some of the important microstructural phenomena involved at each of the stages of processes in a typical C-Q-T process chain. These phenomena can be modeled with the help of appropriate mathematical models. This section details the mathematical models used in the present work to capture microstructural physics of the processes as modeled in this work.
Austenitization During Heating
Austenitization kinetics during initial heating are not modeled in this work and it is assumed that austenitization gets completed on reaching AE3 temperature. AE3 is calculated using the following expression that takes into account the chemical composition of the steel [29]:
where the amount of each alloying element is expressed in weight percent (wt%). This equation was chosen as it incorporates the effect of large number of alloying elements on AE3 predicition.
Grain Growth During Carburizing
The grain growth in the steel during carburizing is modeled with the help of chemical composition-dependent power law expression, given in Eq. 2, developed by Lee et al. [30] for low alloyed steels:
where D is the austenite grain diameter in μm, t is the time in seconds, Ko is pre-exponential constant with a value of 76,671, n is the time exponent and has the value of 0.211, R is the gas constant in J/mol. K, T is the temperature in °C and Q is the activation energy for grain growth in J/mol, given in terms of chemical composition as:
where the amount of each alloying element is expressed in wt%.
Equation 2 gives a grain size evolution under isothermal condition. Therefore, in order to use it for the non-isothermal conditions of industrial practices such as heating and holding, we need to convert this equation in differential form, expressed as:
Carbon Diffusion During Carburizing
The flux of carbon atoms from the carbon-rich atmosphere to the surface of steel specimen is modeled by the equation:
where Cp is the carbon potential of the atmosphere, Cs is the carbon concentration at the steel surface at any point of time and β is the mass transfer coefficient. The typical values of β are reported to lie in between 2 × 10−5 and 2 × 10−4 cm/s for a temperature regime of 800 − 1000°C [31]. Carbon diffusion inside the bulk of the steel is described by Fick’s first law given by:
where J is the flux of the carbon atoms, D is the diffusivity of the steel, and C is the carbon concentration at any point in the steel. Under transient state, the rate of carbon concentration build-up at any point inside the steel specimen is described by Fick’s second law:
Assuming no soot formation at the steel surface, we can define the boundary condition as a mass balance at the steel surface given by:
Diffusivity (m2/s) is calculated using the temperature (T) and carbon % (C)-dependent empirical expression given by Tibbets [32], which is modified to incorporate the effect of alloying elements, as suggested by Neuman and Person [33]:
where R is the gas constant in cal/mol.K, T is in °C and q is given as:
where the amount of each alloying element is expressed in wt. %.
Phase Transformation During Quenching
Quenching from the austenitizing temperature results in the decomposition of austenite to give diffusional as well as non-diffusional (martensitic) phase transformation products depending upon the cooling rate experienced by the quenched part. Diffusional phase transformations during quenching are modeled using a semi-empirical model given by Li et al. [12]. This model expresses the basic kinetic equation for the rate of formation of each diffusional phase transformation product in the form:
where \( \frac{dX\ }{dt} \)is the rate of formation of any product phase, f1(C′) is a function dependent on steel composition (C′), f2(G) is a function dependent on prior austenite grain ASTM Number (G), f3(T) is a function dependent on temperature (T), and f4(X) is a function of already formed normalized fraction (X) of the product phase (the actual or true phase fraction, denoted as X0, of a product phase at a temperature is normalized with respect to the maximum amount of it that can form at that temperature). The actual expressions of these functions for each transformation product (ferrite, pearlite, and bainite), and the methodology of implementation of these kinds of models are present in the literature [12, 34] and hence shall not be reproduced here.
Below M S (martensite-start) temperature, all the diffusive transformations stop and only the martensitic (non-diffusional) transformation takes place. The martensitic transformation is modeled using the Koistinen-Marbuger Eq. [35] given as:
where \( {X}_m^0 \) is the true fraction of martensite that will form at temperature T, and \( {X}_{a- Ms}^0 \) is the austenite true fraction retained at M S temperature.
The phase evolution equation for a particular phase is applicable for temperatures lower than its transformation start temperatures. Chemical composition-dependent empirical equations, reported in the literature, are used for the upper critical temperature (AE3) [29], eutectoid temperature (AE1) [29] and bainite-start temperature (B S ) [12]. Martensite-start temperature (M S ) used in this study is given in Eq. (13)
where the amount of each alloying element is expressed in wt% and C F is the correction factor in the original Steven-Haynes equation [36] of Ms temperature, as suggested by Geoffrey Parrish [37] for high carbon percentage (i.e. for carburized steels). Based on the values of the correction suggested, the expression for C F was formulated as follows:
Figure 2 shows the comparison of C F , calculated from Eq. (14), with the values suggested in the literature [37].
Comparison of the calculated and suggested correction factor (C F ) in M S temperature for high carbon percentage [37]
Hardness After Quenching
Phase fractions calculated at the end of quenching simulation are used to calculate as-quenched hardness using the following chemical composition-dependent expressions of individual phase hardness values, given by Maynier et al. [38]:
where HVa − f − p, HV b , and HV m are the vickers hardness values of austenite-ferrite-pearlite mixture, bainite, and martensite, respectively, and V r is the cooling rate at 700 °C in °C/hr.
Equations (15) to (17) can be used to find the total as-quenched hardness at a material point (HV) with the help of individual phase fractions, using law of mixture as follows:
where \( {X}_a^0 \), \( {X}_f^0 \), \( {X}_p^0 \), \( {X}_b^0 \), and \( {X}_m^0 \) are the true fractions of austenite, ferrite, pearlite, bainite, and martensite, respectively.
Hardness After Tempering
In the present study, in order to model the effect of tempering process, an empirical approach is adopted to predict the hardness values of tempered martensite. Carburized-quenched automotive components are normally tempered in the temperature regime of 150–200 °C for 1–2 h [39]. In this temperature-time regime, only the first stage of tempering is active and the only microstructural change that takes place is tempering of martensite to yield low carbon martensite with carbide precipitation [37]. Since only martensite is affected for the said temperature-time regime of tempering, it is assumed that the rest of the phases do not change in their properties. In this indirect approach, tempering is not explicitly modeled as a physical phenomenon; however, the effect of the tempering process on as-quenched martensite hardness values is taken into account in order to predict important parameters such as total tempered hardness and case depth. As per this approach, the hardness of tempered martensite (\( {HV}_m^T \)) at a given tempering temperature (T t ) and tempering time (t t ) is predicted from as-quenched martensite hardness (HV m ), by combining the concept of Jaffe-Holloman time temperature equivalence [40] and the experimental data for tempered-martensite hardness values for different steels and tempering temperatures [41].
Jaffe-Holloman [40] time temperature equivalence concept states that if the microstructure mechanism of tempering remains the same, for the same tempered hardness value, the Jaffe-Holloman parameter remains constant, as follows:
where, T is any tempering temperature in °C, t is the tempering time in hours, and K is a material constant dependent on carbon content (C) as follows [40]:
Using this concept, first, an equivalent tempering temperature (Teq) is calculated that would result in the same tempered hardness value under 1 h of tempering, as under the required T t tempering temperature (°C) for t t tempering time (hours). Thus, using Eq. (19), Teq(°C) can be calculated as follows:
Next, the tempered martensite hardness value for Teq tempering temperature and 1 h tempering time needs to be calculated. For this, we use the data reported by Grange et al. [41], in which the hardness of martensite, tempered at different tempering temperatures for 1 h, for varying carbon steels, is given along with the as-quenched hardness. The data is used to formulate the factor f which is the ratio of tempered hardness (\( {HV}_m^T \)) and as-quenched hardness (HV m ) values, and denotes the factor by which the hardness of the as-quenched martensite scales-down during tempering. Mathematically, the relationship between\( {HV}_m^T \), HV m , and f is as follows:
Equation (23) gives the functional form of f obtained from the data, as follows:
where T is the tempering temperature (°C) at which we want to calculate f, and C is the carbon content of the steel (wt%). Figure 3 shows the comparison of experimental data [41] with tempered hardness values predicted using Eqs. (22) and (23) for different tempering temperatures and different C steels.
Comparison of the experimental (discrete points) and calculated (solid lines) hardness values for martensite for different carbon (%) steels tempered for 1 h [41]
Using Eq. (23), value of f is calculated at Teq temperature, which in turn is used in Eq. (22) to calculate tempered martensite hardness (\( {HV}_m^T \)) from quenched martensite hardness (HV m ). Since it is assumed that hardness of other phases does not get affected, total tempered hardness (HVT) can thus be calculated using a linear rule of mixture as follows:
The following steps sum up the procedure to calculate HVT:
-
1)
Calculating equivalent temperature (Teq) for the required tempering temperature (T t ), tempering time (t t ), and steel carbon content (C), using Eqs. (20) and (21)
-
2)
Calculating the factor f at Teq temperature for the steel carbon content (C) using Eq. (23)
-
3)
Calculating the tempered martensite hardness value (\( {HV}_m^T \)) from as-quenched martensite hardness value (HV m ) and factor f using Eq. (22)
-
4)
Finally, calculating the total tempered hardness using Eq. (24)
Since most of the industrial standards for hardness are expressed in the Rockwell scale, the following expression, reported in the literature [42], was used to convert the Vickers hardness (HV) values to Rockwell scale (HRc):
Thermal Modeling
The temperature field inside the steel part during the entire process chain is modeled using Fourier’s 3-D heat conduction equation, given in Eq. (26), under convective and radiative boundary conditions of heating/cooling, given in Eqs. (27) and (28), respectively:
where ρ is the density, c is the specific heat, k is the thermal conductivity, \( \dot{q} \) is the rate of latent heat evolution, Ψ c and Ψ r are the heat flux due to convection and radiation, respectively, h is the heat transfer coefficient, ε is the emissivity, T m is the medium’s temperature, T s is the component’s surface temperature, and σ is the Stefan-Boltzmann constant. Wherever applicable, the thermo-physical properties are expressed using the rule of mixtures applied to the thermo-physical properties of the individual phases as follows:
where, P(T) represents any temperature-dependent thermo-physical property (ρ, c, and k), P(T) k is the temperature-dependent thermo-physical property of any kth phase and \( {X}_k^0 \) is its true fraction.
FEM Implementation and Integration
The individual process models of carburizing, quenching and tempering were implemented in a FEM framework on a commercial suite ABAQUS™ with the aid of different user-subroutines. Carburizing was modeled as a thermal and mass-diffusion problem. The heating cycle of the carburizing process is modeled as a thermal analysis simulation, the results from which are fed into a separate mass-diffusion analysis in a sequential fashion. Grain size evolution during heating is calculated at each integration as a state variable using a user-subroutine, taking the temperature results from FEM as input. Carburizing boundary condition, given in Eq. (5), is modeled using the subroutine DFLUX. Quenching is modeled as a thermal analysis problem with phase transformation kinetics modeling, thermo-physical properties assignment, and hardness calculations done through user-subroutine UMATHT. The individual phase fractions and as-quenched hardness values are calculated as solution-dependent state variables. The heat transfer coefficient for the quenching media is modeled as an interaction property. The tempered hardness model is also implemented in UMATHT which uses the individual phases’ as-quenched hardness values and calculates the tempered hardness as a state variable for the required tempering conditions.
After implementing individual models, the models are integrated as a process chain by establishing the information flow from one process model to other. This is realized with the help of ABAQUS™ python scripting which enables extraction of the key output results from one simulation which are then read as the starting state variables values for the next simulation. Figure 4 shows the sequence of the process simulations with their associated information flow.
Validation of Models
Once the individual process models are implemented and their integration is set up, they are validated against various experimental results reported in the literature. Validations were carried out not only for the individual process models but also for their integration. This section describes three such validations. The reported validations include carburizing validation, carburizing-quenching validation, and finally carburizing-quenching-tempering validation. The chemical composition of the steels and their corresponding heat treatment cycle details are reported in their respective cited references and hence not reproduced here.
Validation 1 (Carburizing)
In order to validate the carburization model, the carburizing process of a gear ring of SCR420 steel, as reported by Kim et al. [4], is modeled. Authors reported the carbon profile along the radial section of the carburized gear ring, measured using a glow discharge spectrometer. In order to validate the carburizing model, carburizing simulation was carried out using an axi-symmetric FEM model of the ring of the same dimensions and under the same carburizing cycle as reported. Figure 5a shows the ring geometry highlighting the axi-symmetric section modeled for this study. Figure 5b shows the comparison of final carbon profile calculated along the radial section of gear ring and the experimental measurement. The comparison shows a very good match between the simulation and experimental results.
Details of validation 1. a Geometry of the gear ring used in this study with shaded portion denoting the modeled part. b Comparison of simulation results and experimental values of carbon profile obtained after carburizing cycle [4]
Validation 2 (Carburizing-Quenching)
For validating the modeling of C-Q process chain, one of the benchmark problems of gear ring of JIS-SCR420H steel, reported by Inoue et al. [23] was used. The authors have reported the experimental measurement of the Vickers hardness along the radial section of gear ring, after the carburizing-quenching treatment cycle. In order to model the process chain, an axi-symmetric FEM model of the gear ring, of the reported dimensions, was used. Figure 6a shows the ring geometry highlighting the axi-symmetric section modeled for this study. The carbon distribution extracted after carburizing simulation act as input for quenching simulation for modeling phase transformations. Heat transfer coefficient for the quenching medium was taken from the work of Schichino [43]. Figure 6b shows the variation of calculated quenched hardness along the depth of the ring and its comparison with the experimental measurements. The comparison shows a good agreement between the two.
Details of validation 2. a Geometry of the gear ring used in this study with shaded portion denoting the modeled part. b Comparison of simulation results and experimental values of quenched hardness obtained after carburizing-quenching cycle [23]
Validation 3 (Carburizing-Quenching-Tempering)
The integrated C-Q-T simulation setup was tested for an experimental work as reported by Medlin et al. [44], wherein the modified bending fatigue specimens of SAE 4320 steel are carburized at different carbon potentials (C p ), followed by quenching and tempering. The paper reports the measured surface hardness values, retained austenite percentage and the case depth obtained for each of the carbon potential after the complete heat treatment cycle. In order to model the entire heat treatment process chain, a half 2D FEM model of the section of the fatigue specimen was used. Figure 7 shows the geometry of the model used along with the dimensions (with shaded portion representing the modeled half-part).
Table 1 shows the comparison of the predicted and the reported experimental values of surface hardness, retained austenite, and case depth, for the C p values of 0.85 and 1.05. The predicted results are well in accordance with the experimental results.
Based on these validations, we can conclude that the individual process models as well as their integration capture the essential microstructural physics of the process chain well. The key predictions at the end of each individual process model are found to be matching well with their respective experimental measurements thereby suggesting the importance of integrated modeling of process chain. Since the validations involve different compositions of carburizing grade steels and process conditions, it has been found that the various material models used in this work are in general applicable for carburizing grade steels and for typical C-Q-T process conditions of gears. However, it is to be noted that these material models, taken from the literature as discussed earlier, are empirical or semi-empirical in nature and hence will have limitations of applicability ranges. The individual cited references of these models provide further details on these aspects. Although the authors have found that these models, in general, predict reasonable results for processing conditions of gear steels, yet it may need further validations and enhancements. However, since the present work focusses on establishing an ICME framework for gears, this aspect has not been investigated further.
Application for ICME Workflow for Gears
After establishing the integration of individual process models and validating the predictions of integration, all the operations of pre-processing, post-processing, and information transfer between FEM simulations were automated by a main wrapper program. This enabled the conversion of the entire integrated process chain models into a manufacturing module to be used in an ICME workflow of automotive gears production. When in contact with mating gear, a gear tooth experiences maximum contact stresses at the pitch point and maximum bending stresses at the root point. Hence, both these regions are critical and the material properties at these regions primarily govern the total service life a gear. Moreover, both the case depth and surface hardness values are important design considerations for high fatigue and pitting life of the gears. Such a module takes the gear geometry mesh, chemical composition of the steel, and process set-points as inputs and gives case depths and hardness values as outputs for both pitch and root region. Figure 8 broadly shows the structure of the automated C-Q-T module. The module can then be used in establishing an ICME workflow as shown in Fig. 9. In a traditional design and development approach for products, the stages of geometric design, material selection, and manufacturing have remained largely disjointed and independent of each other. The decision made at any such stage is not fed back to review the decisions made for the other stages. The proposed ICME workflow would enable a closer collaboration between the geometric design, material selection, and manufacturing by letting the manufacturers explore each of the operation in relation to other, thereby enhancing the design space and enabling them to find the most suitable combination of the three operations [45].
In order to demonstrate the application of the C-Q-T module in enabling ICME for automotive gears, a typical case of manufacturing of automotive spur-gear is chosen as an example in this work. Following sub-sections highlight the utility of this module for design and manufacture of automotive gears.
Manufacturing Design
For demonstrating the manufacturing-process design capabilities of the module, heat treatment design of 8620 steel spur-gear was considered. Figure 10 shows details of the geometry of the gear tooth and Table 2 shows the chemical composition of the 8620 steel used in this study.
As per the gear design rules, the minimum case depth requirement for a typical gear design of 4 mm module is around 0.6–0.7 mm [39, 46], surface hardness requirement is around 58–62 HRc [46], and core hardness requirement is 32–48 HRc [39]. Various heat treatment cycles with different combinations of process parameters were simulated for the gear tooth in order to achieve the desired hardness and case depth requirements. Figure 11 shows the schematic of the heat treatment cycle considered in this work along with the various process parameters used in this study. In order to reduce the process design space, few process parameters are set as fixed values, as mentioned in Fig. 11 caption.
Schematic of the heat treatment cycle used in this study highlighting the process parameters. T C = carburizing temperature, T D = diffusion temperature, T T = tempering temperature (170 °C), T Q = quenching temperature (60 °C), T A = ambient temperature (25 °C), C p = carbon potential, t C = carburizing time, t D = diffusion time, t T = tempering time
Consider four such C-Q-T heat treatment cycle schedules HT1, HT2, HT3, and HT4, the process set-points of which are mentioned in Table 3.
Figure 12 shows the result of the final carbon, phase fractions, and tempered hardness profile obtained for these four C-Q-T cycles in the pitch region. The carbon profile in each case governs the final phase fraction obtained at the end of quenching, which along with tempering process conditions govern the final tempered hardness profile. The phase fraction profile predominantly show martensite and austenite phase formation near the pitch surface region. This is because the high amount of carbon at the surface leads to austenite stabilization that resulted in retained austenite after quenching along with martensite.
On comparison, it can be seen that relatively less carburizing time for HT1 schedule results in low carbon diffusion and hence low tempered hardness. As a result, HT1 does not meet the case depth and hardness requirement. In order to achieve the required specifications, the carburizing time is increased from 4 to 6 h (HT2). Because of this, the hardness and the case depth achieved in the case of HT2 is as per the requirement. However, in industry, increasing the carburizing time will result in the increase in cycle time of the heat treatment schedule, leading to an increase in the cost and time of the production cycle. In order to tackle this, another heat treatment cycle (HT3) is tried wherein the carburizing time is still 4 h; however, the carburizing temperature is increased from 900 to 950 °C. Increasing the carburizing temperature leads to an increased diffusivity of the carbon, thereby enabling a higher case depth at the same cycle time. Although HT3 can be the required heat treatment cycle, increasing the carburizing temperature can lead to various other problems such as increased production cost due to higher furnace operating temperatures, higher distortion during quenching, excessive grain growth, etc. Thus as an alternative, HT4, schedule is tried with a lower diffusion temperature of 850 °C. It can be seen that decreasing the diffusion temperature does not lead to a significant effect on case depth and thus the requirements are still met. Figure 13 shows the contour plot of the final carbon, martensite fraction and tempered hardness distribution in the gear tooth for HT4 schedule. Table 4 summarizes the comparison of the final results obtained from C-Q-T manufacturing module for these schedules. It can further be seen from Table 4 that for the same heat treatment schedule, the case depth for the root is always lower than the case depth at pitch. This is because of the effect of the curvature at the root region which leads to lower carbon diffusion and hence lower hardness. This has also been experimentally observed and reported [39, 47].
Since carburizing process set-points effect the final properties of the gear after quenching and tempering, this study shows that an integrated modeling approach is thus necessary to capture the effect of all the processing conditions on the final microstructure and properties of the gear. This necessity is also strengthened by the fact that few heat treatment cycles, even though having different process set-points, met the required specifications. Since we have modeled the process chain in an integrated fashion, we are able to explore the design space of process set-points in relation to each other and arrive at required properties even with different process parameters. To demonstrate it further, Table 5 shows the predictions of some of the other heat treatment cycle schedules that resulted in the required properties. Thus, it can be concluded that such an integrated modeling approach not only helps designers to capture the effect of the processing history on the properties thereby making the predictions more accurate, but also enables them to explore vast manufacturing design space to choose the most suitable processing conditions that yields the desired properties under the plant constraints.
Material Selection and Design
Since all the material models are chemical composition based, thus apart from manufacturing process design, such a methodology can also enable material selection for the required gear geometry and heat treatment. In order to demonstrate this, the HT4 heat treatment cycle for two other typical gear steels viz. 4130 and 4320 were simulated and compared with 8620 steel. 4130 is a high chromium alternative of 8620 steel and 4320 is a high nickel alternative. The composition of these two steels is shown in Table 6.
Table 7 shows the comparison of case depth and hardness values predicted for the three steels. It can be seen that selection of 4130 steels results in lesser case depth but higher surface hardness values for the gear. Such a gear will have a higher wear resistance due to higher surface hardness. This prediction is very well in accordance with the findings of Lyakhovich et al. [48] who studied the effect of chromium content on the carburized steels and found the case depth to decrease and wear resistance to increase with increase in chromium content. On the other hand, 4320 steel has slightly lower surface hardness but higher case depth and core hardness as compared to 8620 steel.
It can be seen that for the same heat treatment and gear geometry, different steel selections can lead to different properties. Moreover, it can also be seen that for the same design and process selection, there can be more than one material that can result in the required properties (for e.g., 8620 and 4320 steel in the above study). In such a case, the decision for material selection can be made on other industrial constrains such as cost or other properties’ requirement. Thus, such a composition-dependent integrated modeling approach not only helps in finding the material composition that results in required properties, but also helps in exploring various composition systems that can meet the property specification thereby, enabling the designer to choose the most suitable material for the application.
Finally, this module can also be helpful in the geometrical design of the gears. A typical example would be deciding the face width of the gear. For automotive manufacturers, size and weight of the components is always a constraint and reducing the face-width of the gear can contribute to size and weight reduction. As per the AGMA strength and stress equations [49], optimizing the surface and core hardness of the gear with the help of the C-Q-T module can lead to an increase in the allowable stresses for both bending and contact, thereby providing the opportunity to reduce the face-width of the gear for the same load bearing capacity.
Summary and Conclusion
In this paper, an integrated approach of modeling carburizing, quenching, and tempering processes by capturing the essential material physics in each process, enabled by flow of appropriate information from one simulation to another, has been established. After validating the material models and the integrated setup for various validation problems, the approach is automated and is used as a module for establishing the ICME approach for production of automotive gears. The applicability of the module is demonstrated by addressing the typical scenarios of the manufacturing design, material selection stages in the design and development cycle of automotive gear. These demonstrations bring out the necessity and the importance of modeling the entire process chain in an integrated fashion, and its importance in establishing the ICME approach that can enable a closer collaboration between geometrical, material, and manufacturing design stages. The ability to accurately predict the effect of change in any of the process chain parameters on the final properties of the product opens up enormous opportunities for the product designer to tailor the microstructure and the final properties of the product in a more optimized and an efficient manner. Moreover, such an approach also provides the window to enable the inverse design of the manufacturing route for the desired gear properties [50]. Although the current work focusses on gear manufacturing, the integrated methodology developed in this work can as well be used for other components such as shafts, pinions, and bearings. As a further work, the methodology is currently being extended to include the prediction of residual stress and distortion and its subsequent effect on the performance of gears under fatigue loading. The integrated methodology and module developed in this paper is intended to become the integral part of an ICME platform for designing and manufacturing of finished and semi-finished components [51].
References
Karabelchtchikova O, Maniruzzaman Md., Sisson Jr. RD. (2006) Carburization process modeling and sensitivity analysis using numerical simulation. In: Proceedings of materials science and technology 2006 conference, Cincinnati, pp 375–386
Kula P, Pietrasik R, Dybowski K (2005) Vacuum carburizing process optimization. J Mater Process Technol 164–165:876–881
Stasiek M, Öchsner A (2006) Numerical simulation of carburization and decarburization profiles in steels. Defect Diffus Forum 258–260:366–371
Kim DW, Cho YG, Cho HH, Kim SH, Lee WB, Lee MG, Han HN (2011) A numerical model for vacuum carburization of an automotive gear ring. Met Mater Int 17(6):885–890. https://doi.org/10.1007/s12540-011-6004-x
Jung M, Oh S, Lee YK (2009) Predictive model for the carbon concentration profile of vacuum carburized steels with acetylene. Met Mater Int 15(6):971–975. https://doi.org/10.1007/s12540-009-0971-1
Johnson WA, Mehl RF (1939) Reaction kinetics in process of nucleation and growth. Trans AIME 135:416–442
Nagasaka Y, Brimacombe JK, Hawbolt EB, Samarasekera IV, Hernandez-Morales B, Chidiac SE (1993) Mathematical model of phase transformations and elastoplastic stress in the water spray quenching of steel bars. Metall Mater Trans A 24(4):795–808. https://doi.org/10.1007/BF02656501
Woodard PR, Chandrasekar S, Yang HTY (1999) Analysis of temperature and microstructure in the quenching of steel cylinders. Metall Mater Trans B 30(4):815–822. https://doi.org/10.1007/s11663-999-0043-4
Gur CH, Tekkaya AE (2001) Numerical investigation of non-homogeneous plastic deformation in quenching process. Mater Sci Eng A 319–321:164–169
Kang SH, Im YT (2005) Three-dimensional finite-element analysis of the quenching process of plain-carbon steel with phase transformation. Metall Mater Trans A 36(9):2315–2325. https://doi.org/10.1007/s11661-005-0104-5
Buchmayr B, Kirkaldy JS (1990) Modeling of the temperature field, transformation behavior, hardness and mechanical response of low alloy steels during cooling from the austenite region. J Heat Treat 8(2):127–136. https://doi.org/10.1007/BF02831633
Li MV, Niebuhr DV, Meekisho LL, Atteridge DG (1998) A computational model for the prediction of steel hardenability. Metall Mater Trans B 29(3):661–672. https://doi.org/10.1007/s11663-998-0101-3
Sharma RC (2003) Principles of heat treatment of steels. New Age International
Perez M, Sidoroff C, Vincent A, Esnouf C (2009) Microstructural evolution of martensitic 100cr6 bearing steel during tempering: from thermoelectric power measurements to the prediction of dimensional changes. Acta Mater 57(11):3170–3181. https://doi.org/10.1016/j.actamat.2009.03.024
Denga X, Ju D (2013) Modeling and simulation of quenching and tempering process in steels. Phys Procedia 50:368–374. https://doi.org/10.1016/j.phpro.2013.11.057
Jung M, Lee SJ, Lee YK (2009) Microstructural and dilatational changes during tempering and tempering kinetics in martensitic medium-carbon steel. Metall Mater Trans A 40(3):551–559. https://doi.org/10.1007/s11661-008-9756-2
Shi W, Yao KF, Chen N, Wang HP (2004) Experimental study of microstructure evolution during tempering of quenched steel and its application. Trans Mater Heat Treat 25(5):736–739
Zhang Z, Delagnes D, Bernhart G (2004) Microstructure evolution of hot-work tool steels during tempering and definition of a kinetic law based on hardness measurements. Mater Sci Eng A 380(1–2):222–230. https://doi.org/10.1016/j.msea.2004.03.067
Wan N, Xiong W, Suo J (2005) Mathematical model for tempering time effect on quenched steel based on Hollomon parameter. J Mater Sci Technol 21(6):803–806
Zabett A, Azghandi SHM (2012) Simulation of induction tempering process of carbon steel using finite element method. Mater Des 36:415–420. https://doi.org/10.1016/j.matdes.2011.10.052
Qin M, Ju DY, Zhang Y, Bian P (2004) Evaluation of microstructure and mechanical properties in case layer of carburizing-quenched scr420 steel by numerical simulation and experimental methods. J Mater Sci Technol 20:41–44
Kang SH, Im YT (2007) Finite element investigation of multiphase transformation within carburized carbon steel. J Mater Process Technol 183(2–3):241–248. https://doi.org/10.1016/j.jmatprotec.2006.10.018
Inoue T, Watanabe Y, Okamura K, Narazaki M, Shichino H, Ju DY, Kanamori H, Ichitani K (2007) Metallo-thermo-mechanical simulation of carburized quenching process by several codes - a benchmark project. Key Eng Mater 340-341:1061–1066. https://doi.org/10.4028/www.scientific.net/KEM.340-341.1061
Sugianto A, Narazaki M, Kogawara M, Shirayori A, Kim SY, Kubota S (2009) Numerical simulation and experimental verification of carburizing-quenching process of SCr420H steel helical gear. J Mater Process Technol 209(7):3597–3609. https://doi.org/10.1016/j.jmatprotec.2008.08.017
Lingamanaik SN, Chen BK (2012) The effects of carburising and quenching process on the formation of residual stresses in automotive gears. Comput Mater Sci 62:99–104. https://doi.org/10.1016/j.commatsci.2012.05.033
Liu CC, Xu XJ, Liu Z (2003) A FEM modeling of quenching and tempering and its application in industrial engineering. Finite Elem Anal Des 39(11):1053–1070. https://doi.org/10.1016/S0168-874X(02)00156-7
Rajeev PT, Jin L, Farris TN, Chandrasekar S (2009) Modeling of quenching and tempering induced phase transformations in steels. J. ASTM Int 6(5):JAI102095
Sarmieno GS, Morelli MA, Cuyas JC, Ledesma AI, Solari MJA (1999) Tratinox: a finite element model of quenching and tempering of stainless steels. In: Totten GE, Liščić B, Tensi HM (eds) Proceedings of the 3rd international conference on quenching and control of distortion. ASM International, Materials Park, Prague
Leslie WC (1981) The physical metallurgy of steels. McGraw Hill, New York
Lee SJ, Lee YK (2008) Prediction of austenite grain growth during austenitization of low alloy steels. Mater Des 29(9):1840–1844. https://doi.org/10.1016/j.matdes.2008.03.009
Munts VA, Baskakov AP (1980) Rate of carburizing of steel. Met Sci Heat Treat 22(5):358–360. https://doi.org/10.1007/BF00693263
Tibbetts GG (1980) Diffusivity of carbon in iron and steels at high temperatures. J Appl Phys 51(9):4813–4816. https://doi.org/10.1063/1.328314
Neuman F, Person B (1968) Beitrag zur metallurgie der gasaufkohlung-Zusammenhang zwischen C-potential der gasphase und der Werkstuckes unter Berichsichtigung der Legierungselemente. Harterei-Techn. Mitt 23(4):296–310
Nguyen TC, Weckman DC (2006) A thermal and microstructure evolution model of direct-drive friction welding of plain carbon steel. Metall Mater Trans B 37B:275–292
Koistinen DP, Marburger RE (1959) A general equation prescribing the extent of the austenite-martensite transformation in pure iron-carbon alloys and plain carbon steels. Acta Metall 7(1):59–60. https://doi.org/10.1016/0001-6160(59)90170-1
Steven W, Haynes AG (1956) The temperature of formation of martensite and bainite in low-alloy steel. J Iron Steel Inst 183:349–359
Parrish G (1999) Carburizing: microstructures and properties. ASM International, Materials Park
Maynier P, Jungmann B, Dollet J (1978) Creusot-Loire system for the prediction of the mechanical properties of low alloy steel products. In: Doane DV, Kirkaldy JS (eds) Hardenability concepts with applications to steel: proceedings of a symposium held at the Sheraton-Chicago Hotel, October 24–26, 1977. Metallurgical Society of AIME, New York, pp 518–545
Rakhit AK (2000) Heat treatment of gears: a practical guide for engineers. ASM International, Materials Park
Hollomon JH, Jaffe LD (1945) Time-temperature relations in tempering steel. Trans AIME 162(223–249)
Grange RA, Hribal CR, Porter LF (1977) Hardness of tempered martensite in carbon and low-alloy steels. Metall Trans A 8(11):1775–1785. https://doi.org/10.1007/BF02646882
Cojocaru M O, Popescu N, Drugă L (2012) The estimation of the quenching effects after carburising using an empirical way based on Jominy test results. In: Nusheh M, Ahuett HG, Arrambide A (eds) Recent researches in metallurgical engineering—from extraction to forming, InTech, pp 91–122
Hayato S (2005) Construction of heat treatment database and enhancement of simulation technique. KOMATSU Tech Rep 51(155):1–9
Medlin DJ, Cornelissen BE, Matlock DK, Krauss G, Filar RJ (1999) Effect of thermal treatments and carbon potential on bending fatigue performance of SAE 4320 gear steel. SAE Technical Paper 1999-01-0603. Doi: https://doi.org/10.4271/1999-01-0603
Gautham BP, Kulkarni N, Khan D, Zagade P, Reddy S, Uppaluri R (2013) Knowledge assisted integrated design of a component and its manufacturing process. In: Li M, Campbell C, Thornton K, Holm E, Gumbsch P (eds) Proceedings of the 2nd world congress on integrated computational materials engineering (ICME). John Wiley & Sons, Inc., Hoboken. https://doi.org/10.1007/978-3-319-48194-4_47
Radzevich SP (2012) Dudley’s handbook of practical gear design and manufacture. CRC Press, Boca Raton. https://doi.org/10.1201/b11842
Davis JR (2005), Gear materials, properties, and manufacture. ASM International, Materials Park
Lyakhovich LS, Voroshnin LG, Rostovtsev AN (1975) Effect of chromium on the depth and properties of the carburized case on low-carbon steel. Met. Sci. Heat Treat. 17(8):648–650. https://doi.org/10.1007/BF00664307
Budynas RG, Nisbett KJ (2008) Shigley’s mechanical engineering design. McGraw Hill, New York
Kulkarni N, Gupta R, Khan D, Gautham BP, Allen JK, Panchal J, Mistree F (2014) Inverse Design of Manufacturing Process Chains. In: Proceedings of the ASME 2014 International design engineering technical conferences & computers and information in engineering conference, ASME, New York
Gautham BP, Singh AK, Ghaisas SS, Reddy SS, Mistree F (2013) PREMΛP: a platform for the realization of engineered materials and products. In: Chakrabarti A, Prakash RV (eds) ICoRD’13, lecture notes in mechanical engineering. Springer, Chennai. https://doi.org/10.1007/978-81-322-1050-4_104
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Khan, D., Gautham, B. Integrated Modeling of Carburizing-Quenching-Tempering of Steel Gears for an ICME Framework. Integr Mater Manuf Innov 7, 28–41 (2018). https://doi.org/10.1007/s40192-018-0107-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40192-018-0107-x