Abstract
Introduction
SB11 was recently approved as a ranibizumab biosimilar by the US Food and Drug Administration (FDA) and the European Commission (EC) as a therapy for retinal vascular disorders under the brand name Byooviz™. This study was performed to assess the analytical similarity between SB11 and reference products from the European Union (EU-ranibizumab) and United States (US-ranibizumab).
Methods
A comprehensive structural, physicochemical, and biological characterization was performed utilizing state-of-the-art analytical methods. Comparisons included the following: primary structure related to amino acid sequence and post-translational modifications; higher order structure; product-related substances and purity/impurity including size and charge variants. In addition, biological characterization included a series of mechanism of action (MoA)-related bioassays such as vascular endothelial growth factor (VEGF)-A binding assay (VEGF-A 165 and its isoforms), cell-based VEGF-A 165 neutralization assay, and anti-proliferation assay using human umbilical vein endothelial cells (HUVEC).
Results
The amino acid sequence of SB11 was identical to that of reference products, and post-translational modification profiles and higher order structures of SB11 were shown to be indistinguishable from the reference products. Product-related size and charge variants and aggregates were also similar. Using a broad range of VEGF-related functional assays, we demonstrated that SB11 has similar biological properties to reference products in VEGF-A binding activities (VEGF-A 165 and isoforms (VEGF-A 110, VEGF-A 121, and VEGF-A 189)), VEGF-A 165 neutralization, and HUVEC anti-proliferation. Overall, SB11 exhibits high similarity compared to EU/US-ranibizumab.
Conclusion
Based on the comprehensive analytical similarity assessment, SB11 is highly similar to the EU/US-ranibizumab with respect to structural, physicochemical, and biological properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
SB11 has been developed as a ranibizumab biosimilar and approved by the US Food and Drug Administration (FDA) and European Commission (EC). |
SB11 demonstrated high similarity with reference products (EU/US-ranibizumab) in terms of structural, physicochemical, and biological properties. |
Introduction
The VEGF family includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PLGF) controls angiogenesis and vascular permeability. In particular, VEGF-A, which is over-expressed in retinal cells, is a critical mediator of neovascular ocular diseases due to ocular angiogenesis and vascular permeability. Therefore, anti-angiogenic therapy by VEGF-A neutralization is the standard of care for treating pathological neovascularization diseases [1,2,3,4,5]. Ranibizumab inhibits the interaction between VEGF-A and VEGF-A receptors (VEGFR1 and VEGFR2) on the surface of endothelial cells to prevent VEGF-A signaling, thereby inhibiting cell functions such as proliferation, permeabilization, migration, and angiogenesis [6].
Lucentis® is a humanized anti-VEGF antibody fragment (Fab) approved by the US FDA and EC and other countries for the treatment of retinal vascular diseases that cause visual loss or blindness due to angiogenesis promotion in the eyes. The indications of retinal vascular diseases include neovascular age-related macular degeneration (nAMD), proliferative diabetic retinopathy (PDR), diabetic macular edema (DME), myopic choroidal neovascularization (CNV), and macular edema following retinal vein occlusion (RVO) [7, 8]. SB11 has been developed as a biosimilar of Lucentis® and obtained marketing authorization in the EU and US in September 2021 as the first biosimilar of Lucentis® [nAMD, PDR, DME, CNV, and RVO (branch RVO or central RVO) for EU and nAMD, CNV, and RVO for US] [9, 10].
Biosimilar development requires a stepwise approach to demonstrate similarity between a proposed biosimilar and an authorized original biological medicine (reference product). The foundation of the biosimilarity assessment is a comprehensive structural and functional similarity evaluation using state-of-the-art analytical techniques [11,12,13,14]. To determine the analytical similarity of SB11 and EU/US-ranibizumab, the products were characterized in terms of the primary structure, higher order structures, and physicochemical and functional properties. In this study, a pre-defined quality range approach with EU/US-ranibizumab or side-by-side comparison was applied according to the quality attribute characteristics. The data presented in this journal demonstrate that the comprehensive characteristics of SB11 and EU/US-ranibizumab are very similar.
Methods
This article does not contain any studies with human participants or animals performed by any of the authors. All SB11 samples were prepared and experiments conducted aseptically in a biological safety cabinet.
Reference Products
EU/US-ranibizumab, which expired from December 2016 to August 2021, was purchased from local distributors and then stored according to the manufacturer’s instructions.
Peptide Mapping
Peptide mapping was performed to analyze structural integrity, including post-translational modifications and disulfide bond by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC–ESI–MS/MS). To achieve denaturation and reduction, ranibizumab was mixed with 8 M urea and 1 M dithiothreitol; 1 M iodoacetamide was added. The sample was digested with trypsin (11047841001, Roche, Basel, Switzerland), Lys-C (90051, Thermo scientific, Waltham, MA, USA), and Asp-N (11058541103, Roche). For the disulfide linkage analysis, the reducing step was not performed, and the non-reduced samples were digested with trypsin. The digestion samples underwent reverse-phase ultra performance liquid chromatography-mass spectrometry (RP-UPLC-MS) using a BEH300 C18 column (186003687, Waters, Milford, MA, USA) at 60 °C. Peptides were eluted by a linear gradient at a flow rate of 0.3 ml/min for 100 min. Data were collected and processed by MassLynx v4.1 (Waters) and/or BiopharmaLynx v1.2 (Waters).
Circular Dichroism Spectroscopy
For far-UV studies, ranibizumab was dialyzed with 10 mM sodium citrate buffer and diluted with 10 mM sodium citrate. For near-UV studies, the samples were not dialyzed but instead diluted with SB11 formulation buffer. Circular dichroism spectroscopy (CD) measurements were performed using a Chirascan Q100 (Applied Photophysics, Leatherhead, UK) with 0.1-mm pathlength cells for far-UV and 10-mm pathlength cuvette for near-UV. The observed CD data in ellipticity for each sample were blank-subtracted and the average of the triplicate scans was used to make the CD plot. The CDNN algorithm was used to fit the far-UV CD data for prediction of secondary structure.
Fourier Transform Infrared Spectroscopy
The Fourier transform infrared spectroscopy (FT-IR) spectra were recorded from a Nicolet iS50 FT-IR spectrophotometer (Thermo Scientific), equipped with a Smart Orbit diamond ATR accessory and the OMNIC software package for spectrometer control and data analysis. All spectra were acquired at 4 cm−1 resolution between 4000 and 525 cm−1, and a background scan with the matching SB11 formulation buffer was acquired prior to a scan of each sample. Ranibizumab spectra were partitioned into peak areas according to structural contribution and the results averaged over three replicates per sample.
Differential Scanning Calorimetry
Nano-differential scanning calorimetry (DSC) (TA Instruments, New Castle, DE, USA) was used to analyze the melting temperature of samples. The ranibizumab and SB11 formulation buffer were scanned from 15 to 100 °C using a scan rate of 1.5 °C/min. Ranibizumab was diluted with the SB11 formulation buffer prior to the run. To determine the baseline value, the SB11 formulation buffer was loaded into a sample channel and scanned to verify a reproducible baseline for subtraction from sample data. Data were analyzed by TA Instruments NanoAnalyze software.
Hydrogen/Deuterium Exchange-Mass Spectrometry
Hydrogen/deuterium exchange-mass spectrometry (H/DX-MS) was adapted to compare higher order structures between ranibizumab samples. H/DX-MS was initiated by a 1:8 dilution of sample in D2O buffer at intervals of 10 s, 1 min, 10 min, 1 h, and 4 h before quenching and injecting into the mass spectrometer. Peptides were digested on an immobilized pepsin column, and the trapped peptide fragments were eluted by a gradient of 8% to 92% acetonitrile in 15 min. Mass spectra were collected in MSE mode, and data were analyzed by ProteinLynX Global Server™ (Waters) to identify peptides and by DynamX software (Waters) to calculate deuterium uptake and generate butterfly and difference plots.
Size Exclusion-High Performance Liquid Chromatography
Ranibizumab was injected onto a TSK-Gel Super SW2000 SWXL analytical column (0018674, Tosoh, Tokyo, Japan), which was connected to a Waters HPLC system; monitoring was done by UV detection at 280 nm. The flow rate was 0.2 ml/min. Data were acquired and processed by Empower®3 software (Waters).
Capillary Electrophoresis-Sodium Dodecyl Sulfate
Reduced and non-reduced capillary electrophoresis-sodium dodecyl sulfate (CE-SDS) analyses were conducted with a high-performance capillary electrophoresis system (PA800 plus Pharmaceutical Analysis System; SCIEX, Framingham, MA, USA). For the reduced condition, ranibizumab was mixed with a 10-kDa internal standard, SDS-MW sample buffer (A10663, Beckman Coulter, Brea, CA, USA), and 2-mercaptoethanol and then boiled at 70 °C for 5 min. For the non-reduced condition, 2-mercaptoethanol was replaced with iodoacetamide. The sample was electrokinetically introduced onto a capillary (base fused-silica capillary; Beckman Coulter) by applying voltage at -5 kV for 20 s and separated in the capillary cartridge. Electrophoresis was performed at a constant voltage with an applied field strength of -497 V. Data were acquired and processed by 32 Karat software (SCIEX) with integration capabilities.
Imaged Capillary Isoelectric Focusing
Ranibizumab was mixed with pharmalyte 3–10, pharmalyte 8–10.5, 1% methyl cellulose, distilled water, pI 6.61 marker, and pI 9.5 marker. The mixture was loaded onto an iCE3 icIEF instrument (ProteinSimple, San Jose, CA, USA) using a capillary cartridge at 4 °C. Data were acquired by CFR software (ProteinSimple) and processed by Empower®3 software (Waters).
VEGF-A 165/189/121/110 Binding Assay
Relative binding activities of ranibizumab to VEGF-A 165/189/121/110 were measured using an enzyme-linked immunosorbent assay (ELISA). A recombinant human vascular endothelial growth factor (rhVEGF), VEGF-A 165 (293-VE/CF, R&D Systems, Minneapolis, MN, USA), VEGF-A 189 (8147-VE/CF, R&D Systems), VEGF-A 121 (4644-VS/CF, R&D Systems), and VEGF-A 110 (5336-VE-010/CF, R&D Systems), was absorbed onto a 96-well plate. Serial dilutions of ranibizumab bound to rhVEGF followed by the sequential addition of anti-human IgG (Fab-specific)-peroxidase antibody, tetramethylbenzidine (TMB, T0440, Sigma-Aldrich, St. Louis, MO, USA) substrate, and 1 N sulfuric acid. The measurement of the absorbance at a wavelength of 450 nm quantitated the relative binding activity of ranibizumab to rhVEGF. The VEGF binding activity of ranibizumab sample was calculated relative to a reference standard using a four-parameter logistic model [PLA (Parallel Line Analysis); Stegmann Systems GmbH, Rodgau, Germany].
HUVEC Anti-Proliferation Assay (VEGF-A 165)
HUVEC (C2519A, Lonza, Basel, Switzerland) in endothelial cell growth medium (EBM™-2, CC-3156, Lonza) was treated on microplates where VEGF A-165 (293-VE/CF, R&D systems) and serially diluted ranibizumab were pre-incubated and proliferated for 4 days at 37 °C, 5% CO2 incubator. The relative cell proliferation was measured by CellTiter-Blue® cell viability assay kit (G8082, Promega, Madison, WI, USA) using a SpectraMax (M3; Molecular Devices, San Jose, CA, USA). Anti-proliferation activity was calculated relative to a reference standard four-parameter logistic model (PLA; Stegmann Systems GmbH).
VEGF-A 165 Neutralization Assay
Engineered cell lines for VEGF signaling (NFAT-RE-Luc2P/KDR 293 cells, E8510, Promega) in assay media (DMEM, 11995, Gibco, Grand Island, NY, USA) were treated on microplates where VEGF A-165 (293-VE/CF, R&D systems) and serially diluted ranibizumab had been pre-incubated. After cell treatment, the microplate was incubated at 37 °C, 5% CO2 incubator for 3.5–6 h to induce luciferase gene expression by VEGF signaling. The relative VEGF neutralizing activity was measured by a luminescent detection system (Steady-glo®, E2520, Promega) via microplate reader (EnVision, 2104, Perkin Elmer, Waltham, MA, USA). VEGF neutralization activity was calculated relative to a reference standard four-parameter logistic model (PLA; Stegmann Systems GmbH).
Results
To demonstrate the similarity between SB11 and the EU/US-ranibizumab, the analytical characterization was performed using state-of-the-art methods, the primary structure, higher order structure, and physicochemical and functional activities.
The analytical methods utilized for the similarity assessment are summarized in Table 1.
Primary Structure
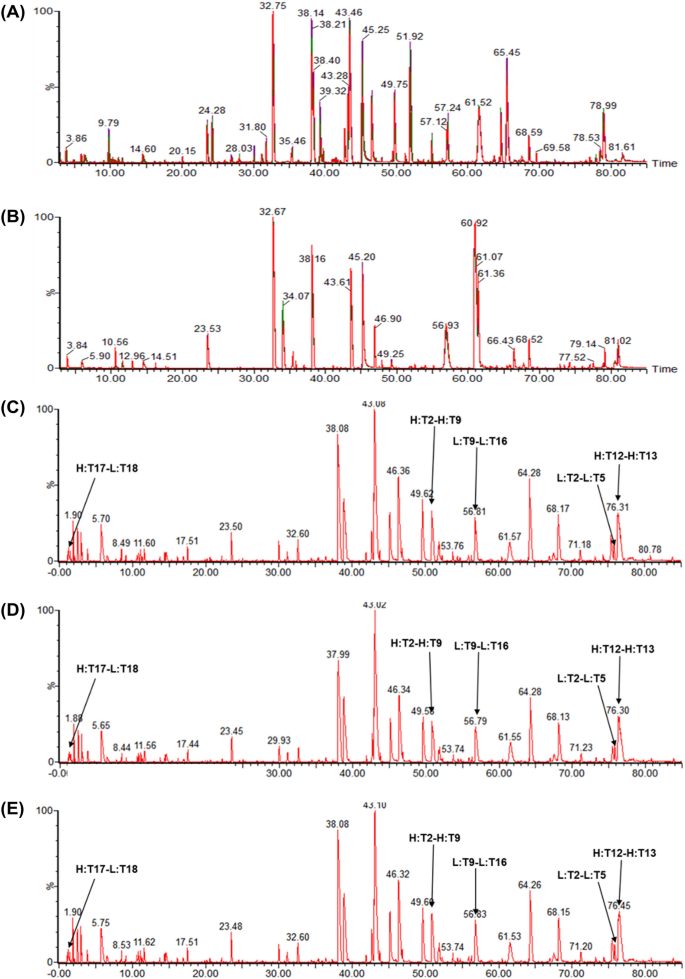
The peptide chromatograms of trypsin or Lys-C digested SB11 and EU/US-ranibizumab showed a highly similar peak profile without missing or additional new peaks with comparative retention time (Fig. 1A, B). In addition, the data confirmed that the amino acid sequences of SB11 and EU/US-ranibizumab were identical and that the sequence coverage by MS/MS was 100% (data not shown). Based on the results of intact mass and peptide mapping analysis, the molecular weights of SB11 and EU/US-ranibizumab were identical (considering assay variability; 0.01% of molecular weight and 30 ppm for intact protein and peptide, data not shown). Sequence variants generated by post-translational modifications such as oxidation and deamidation were identified. The CDR region of Fab contains methionine (Met) residues that are susceptible to oxidation and asparagine (Asn) residues that are susceptible to deamidation [15]. The relative levels of Met oxidation and Asn deamidation were similarly very low (< 0.4%) in all samples (data not shown). Disulfide bond analysis of SB11 and EU/US-ranibizumab under non-reducing condition after trypsin digestion showed that all ten cysteine residues are properly involved in the formation of the five disulfide bonds (Fig. 1C–E, Table 2).
Higher Order Structures
The higher order structures of SB11 and EU/US-ranibizumab were compared using CD, FT-IR spectroscopy, and DSC. The far UV CD spectrum of SB11 and EU/US-ranibizumab showed comparable profiles throughout much of the far-UV region, especially at the local minimum at around 218 nm, which strongly corresponds to the secondary structure of protein folding (Fig. 2A). However, as the curves reach the far-UV region lower than about 210 nm, there was some divergence within samples. In this region, the buffer absorbance and concentration differences can play a role; this non-overlap does not necessarily correlate with a difference in protein folding. In addition, the samples showed comparable near-UV CD profile maxima at 258 nm, 265 nm, 285 nm, and 291 nm, respectively (Fig. 2B). No significant differences between the samples were observed, suggesting the samples share common tertiary structure characteristics. Secondary structure was further identified by FT-IR spectroscopy. The second derivative spectra of SB11 and EU/US-ranibizumab in the amide I band (1600–1700 cm−1) and their distribution into the amide regions support high similarity in secondary structures (Fig. 2C). The DSC thermograms of samples showed highly similar thermal profiles and thermal transition midpoint temperatures, indicating that the thermal stability and conformation of SB11 are highly similar to those of EU/US-ranibizumab (Fig. 2D). Tertiary structure was also determined using H/DX-MS. The dynamics of deuterium uptake over time (10 s to 4 h) revealed high symmetry between SB11 and EU/US-ranibizumab (Fig. 2E, F).
A–D Comparison for higher order profiles of SB11 and EU/US-ranibizumab. A Far-UV CD spectra, B near-UV CD spectra, C second derivative FT-IR spectra, D DSC thermograms. E,F Comparison deuterium uptake profiles of SB11 and EU/US-ranibizumab. E Butterfly plot of SB11 and EU-ranibizumab for heavy chain (top) and light chain (bottom). F Butterfly plot of SB11 and US-ranibizumab for heavy chain (top) and light chain (bottom)
Purity and Impurity
The purity of the products was analyzed via product monomer content and amount of intact Fab, heavy chain, and light chain determined by SE-HPLC and CE-SDS, respectively. In SE-HPLC analysis, all samples showed prominent monomer peaks (Fig. 3A, B). The level of high molecular weight (%HMW) species of SB11 and EU/US-ranibizumab was comparably low (Table 3). The results were confirmed by orthogonal analyses using size exclusion chromatography-coupled multi-angle light scattering (SEC-MALS) and sedimentation velocity analytical ultracentrifugation (SV-AUC) (data not shown). In CE-SDS analysis, the results showed that the electrophoretic profiles of SB11 and EU/US-ranibizumab were similar (Fig. 3C, D). The level of intact Fab species (%Main for non-reduced CE-SDS), heavy chain (%Main 2 for reduced CE-SDS), and light chain (%Main 1 for reduced CE-SDS) in SB11 were similar to those of EU/US-ranibizumab (Table 3).
A, B Comparison of SE-HPLC profiles of SB11 and EU/US-ranibizumab. A Overlay of SE-HPLC profiles, B enlarged view of SE-HPLC profiles, C,D comparison for CE-SDS profiles of SB11 and EU/US-ranibizumab. C Overlay of CE-SDS profiles under non-reduced conditions and D reduced conditions. E,F Comparison of charge variants of SB11 and EU/US-ranibizumab. E Overlay of icIEF profiles, F enlarged view of icIEF profiles
Charge Heterogeneity
To assess charge variants of SB11 and EU/US-ranibizumab, icIEF analysis was performed. The results of icIEF showed that the isoelectric point (pI) values of all samples were identical and their electropherograms were similar (Fig. 3E, F). The results for %Main, %Acidic, and %Basic of all samples were comparable (Table 3). These results have been confirmed by orthogonal analytical method which is cation exchange-high performance liquid chromatography (CEX-HPLC) (data not shown).
Biological Activity
As MoA-related biological activities of ranibizumab reflect physiological and pathological conditions, the VEGF-A 165 binding, VEGF-A 165 neutralization assay and anti-proliferation assay were performed. Additionally, binding activities for VEGF-A isoforms, excluding the VEGF-A 165, were evaluated to demonstrate similarity between SB11 and EU/US-ranibizumab.
MoA-Related Biological Activities
Reference product lots with different expiry dates within the approved shelf life were used for establishing similarity ranges (mean ± 3 SD). SB11 drug products manufactured at commercial scale were compared with EU-ranibizumab and US-ranibizumab for the biological activities. Evaluation of the VEGF-A 165 binding activity by ELISA showed the mean %relative binding activity of SB11, EU-ranibizumab, and US-ranibizumab were 98%, 100%, and 100%, respectively. No meaningful differences between SB11, and EU/US-ranibizumab were observed and VEGF-A 165 binding activities of SB11 were within the similarity range (Fig. 4A). The relative VEGF-A 165 neutralization potency of SB11 was similar to that of EU/US-ranibizumab. Mean %relative potencies of SB11, EU-ranibizumab, and US-ranibizumab were 99%, 100%, and 101%, respectively. In addition, VEGF-A 165 neutralization potencies of SB11 were within predetermined mean ± 3 SD of similarity range (Fig. 4A). For the HUVEC anti-proliferation assay, the mean values of %relative anti-proliferation potencies of SB11, EU-ranibizumab, and US-ranibizumab were 100%, 101%, and 102%, respectively. All SB11 batches also showed relative potencies within the similarity range, demonstrating promising similarity in HUVEC anti-proliferation potency (Fig. 4A). These results support that SB11 is similar to EU/US-ranibizumab in terms of MoA-related biological activities.
Comparison of the biological activities of SB11 and EU/US-ranibizumab. Dotted line shows the similarity range (mean ± 3 SD) of EU-ranibizumab. Solid line shows the similarity range (mean ± 3 SD) of US-ranibizumab®. A MoA-related biological activities; VEGF-A 165 binding (left), VEGF-A neutralization (middle), and HUVEC anti-proliferation (right). B Additional biological activities; VEGF-A 121 binding (left), VEGF-A 110 binding (middle), and VEGF-A 189 binding (right)
Additional Biological Activities
Additional biological activities and VEGF-A 110, VEGF-A 121, and VEGF-A 189 binding activities were assessed of SB11 in a side-by-side comparison. Mean values of %relative VEGF-A 110 binding of SB11, EU-ranibizumab, and US-ranibizumab were 102%, 98%, and 101%, respectively, and those of %relative VEGF-A 121 binding activities were 101%, 103%, and 98%, respectively. In addition, mean values of %relative VEGF-A 189 binding activities of SB11, EU-ranibizumab, and US-ranibizumab were 99%, 97%, and 96%, respectively. No differences were observed between SB11 and EU/US-ranibizumab in the side-by-side comparison; relative VEGF-A 110, VEGF-A 121, and VEGF-A 189 binding activities of SB11 were similar to those of EU/US-ranibizumab (Fig. 4B).
Discussion
A biosimilar needs to demonstrate a high level of similarity with the reference products in terms of structural and functional properties as well as clinical outcomes. The totality of the evidence generated from analytical, functional, non-clinical, and clinical studies is conducted in a stepwise manner. Comparative analytical characterization is the foundation for the biosimilar development. Since our study focuses on the biosimilarity demonstration in respect to structural and functional characterization, other clinical aspects (efficacy, safety, pharmacokinetics, and immunogenicity) of SB11 in Phase 3 study have not been reported and discussed in the study. However, the comprehensive analytical characterization package presented here complements the clinical similarity of SB11 and reference product observed in the comparative clinical trial [16, 17].
For the broad analytical characterization of SB11 as biosimilar of Lucentis®, the quality attributes used for the similarity assessment were ranked (‘tiered’) according their potential impact on PK/PD, safety, efficacy, and immunogenicity [13]. According to quality risk assessment, structural, physicochemical, and functional studies were conducted in line with FDA/EMA standards [12, 13]. For each quality attribute, pre-defined quality ranges were established on the basis of analysis of batches of EU/US-ranibizumab and side-by-side comparisons were performed for similarity demonstration. To establish a quality range, a sufficient number of reference product lots were secured and analyzed over a long period, and the similarity range was statistically established through their quality monitoring.
EU-ranibizumab and US-ranibizumab show comparability in quality aspects (data not shown), and similarity of SB11 to EU/US-ranibizumab was demonstrated in terms of the structural, physicochemical, and biological activities using state-of-the-art methods. For structural integrity, there was no apparent difference between SB11 and EU/US-ranibizumab. Especially, the levels of deamidation and oxidation were important in that these post-translational modifications have potential to affect VEGF-A binding affinity [18]. The results showed the similarity not only for the modification level but also for the VEGF-A binding affinity. No differences were observed in the tertiary structures between SB11 and EU/US-ranibizumab, showing high similarity. Moreover, no relevant differences were observed in terms of purity/impurity and charge variants, strongly supporting the similarity between SB11 and EU/US-ranibizumab. Therefore, the results can support the similarity in structural and physicochemical properties between SB11 and EU/US-ranibizumab.
VEGF-A 165 is the key determinant of MoA of ranibizumab and is most extensively investigated in respect to its function, signaling, expression, and pathological roles. As a potent stimulator of angiogenesis, VEGF-A 165 is considered the prototypical pro-angiogenic VEGF-A isoform [19]. In terms of biological activities, VEGF-A 165 neutralization and HUVEC anti-proliferation were similar for SB11 and EU/US-ranibizumab, and there were no differences in binding activities for VEGF-A 165 and its isoforms (VEGF-A 110, VEGF-A 121, and VEGF-A 189). Taken together, the observed structural, physicochemical, and biological similarities between SB11 and EU/US-ranibizumab are part of the totality of the evidence supporting biosimilarity of SB11 and EU/US-ranibizumab.
Conclusion
SB11 was developed as a ranibizumab biosimilar in accordance with guidelines of international regulatory organizations. Extensive analytical characterization between SB11 and EU/US-ranibizumab was conducted on structural, physicochemical, and biological properties to support biosimilarity of SB11. Analytical characterization results showed that SB11 is highly similar to EU/US-ranibizumab with respect to overall critical and non-critical quality attributes performed.
References
Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76.
Zampros I, Praidou A, Brazitikos P. Antivascular endothelial growth factor agents for neovascular age-related macular degeneration. J Ophthalmol. 2012:319728.
Simons M, Gordon E, Claesson-Welsh L. Mechanism and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17(10):611–25.
Vempati P, Popel AS, Gabhann FM. Extracellular regulation of VEGF: isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. 2014;25(1):1–19.
Ono K, Hsttori H, Takeshita S. Structural features in heparin that interact with VEGF165 and modulate its biological activity. Glycobiology. 1999;9(7):705–11.
Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15(2):171–85.
Lucentis®: EPAR from EMA, Nov 2018. https://www.ema.europa.eu/en/documents/product-information/lucentis-epar-product-information_en.pdf.
Lucentis®: Approval package from FDA, Apr 2017. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/125156s0000_LucentisTOC.cfm.
Byooviz™: EPAR from EMA, Sep 2021. https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-byooviz_en.pdf.
Byooviz™: Approval information from FDA, Sep 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761202s000lbl.pdf.
European Medicines Agency. Guidance for similar biological medicinal products containing monoclonal antibodies: non-clinical and clinical issues, EMA/CHMP/BWP/247713/2012, Jul 2015. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active_en-2.pdf.
European Medicines Agency. Guidance for similar medicinal products containing biotechnology-derived proteins as active substance: quality issues, EMA/CHMP/BMWP/403543/2010, Dec 2014. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active_en-0.pdf.
Food and Drug Administration. Guidance for comparative analytical assessment and other quality-related considerations; draft guidance for industry, CDER/CBER, May 2019. https://www.fda.gov/media/125484/download.
Food and Drug Administration. Guidance for scientific considerations in demonstrating biosimilarity to a reference product; draft guidance for industry, CDER/CBER, Apr 2015. https://www.fda.gov/media/82647/download.
Alam ME, Slaney TR, Wu L, Das TK, Kar S, Barnett GV, Leone A, Tessier PM. Unique impacts of methionine oxidation, tryptophan oxidation, and asparagine deamidation on antibody stability and aggregation. J Pharm Sci. 2020;109(1):656–69.
Bressler SB, Odia I, Maguire MG, Dhoot DS, Glassman AR, Jampol LM, Marcus DM, Solomon SD, Sun JK. Factors associated with visual acuity and central subfield thickness changes when treating diabetic macular edema with anti-vascular endothelial growth factor therapy: an exploratory analysis of the protocol t randomized clinical trial. JAMA Ophthalmol. 2019;137(4):382–9.
Woo SJ, Cho GE, Cho JH. Short-term efficacy and safety of ranibizumab for neovascular age-related macular degeneration in the real world: a post-marketing surveillance study. Korean J Ophthalmol. 2019;33(2):150–66.
Bertolotti-Ciarlet A, Wang W, Lownes R, Pristatsky P, Fang Y, McKelvey T, Li Y, Li Y, Drummond J, Prueksaritanont T, Vlasak J. Impact of methionine oxidation on the binding of human IgG1 to FcRn and Fcγ receptors. Mol Immunol. 2009;46(8–9):1878–82.
Peach CJ, Mignone VW, Arruda MA, Alcobia DC, Hill SJ, Kilpatrick LE, Woolard J. Molecular pharmacology of VEGF-A isoforms: binding and signalling at VEGFR2. Int J Mol Sci. 2018;19(4):1264.
Acknowledgements
The authors thank the quality evaluation team (Samsung Bioepis Co., Ltd.) for their helpful assistance in performing the test.
Funding
Sponsorship for this study and the journal’s Rapid Service Fees were funded by Samsung Bioepis Co., Ltd. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Eunji Kim and Jihyeon Han contributed equally as the first authors, and Yunjung Chae and Hyerim Park contributed equally as second authors. Beom Chan Kim is the corresponding author of this article. Eunji Kim, Jihyeon Han, and Beom Chan Kim performed the collection, analysis and interpretation of the data, drafting of the manuscript, and revision of article. Yunjung Chae and Hyerim Park participated in figure design and manuscript writing. Saerom Kim, Seokkyun Kim, Jungmin Lee discussed the results and reviewed the manuscript.
Disclosures
Eunji Kim, Jihyeon Han, Yunjung Chae, Hyerim Park, Saerom Kim, Seokkyun Kim, Jungmin Lee, and Beom Chan Kim are employees of Samsung Bioepis Co., Ltd.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors. All SB11 samples were prepared and experiments conducted aseptically in a biological safety cabinet.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is licensed under a Creative Commons Attribution-Non-Commercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc/4.0/.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kim, E., Han, J., Chae, Y. et al. Evaluation of the Structural, Physicochemical, and Biological Characteristics of SB11, as Lucentis® (Ranibizumab) Biosimilar. Ophthalmol Ther 11, 639–652 (2022). https://doi.org/10.1007/s40123-022-00453-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00453-7