Abstract
Introduction
Since 2009, the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) surveillance study has been assessing in vitro antibiotic resistance for bacterial isolates sourced from ocular infections in the US. The main goal of this systematic review was to compare in vitro resistance data for ocular pathogens from published US studies with the most recently published data from the ARMOR study (2009–2018) and, where possible, to evaluate trends in bacterial resistance over time over all studies.
Methods
A literature search was conducted using MEDLINE®, BIOSIS Previews®, and EMBASE® databases (1/1/1995–6/30/2021). Data were extracted from relevant studies and antibiotic susceptibility rates for common ocular pathogens (Staphylococcus aureus, coagulase-negative staphylococci [CoNS], Streptococcus pneumoniae, Pseudomonas aeruginosa, and Haemophilus influenzae), longitudinal changes in susceptibility, and multidrug resistance (MDR) were compared descriptively.
Results
Thirty-two relevant studies were identified. High in vitro resistance was found among S. aureus and CoNS to fluoroquinolones, macrolides, and methicillin/oxacillin across studies, with high rates of MDR noted, specifically among methicillin-resistant staphylococci. Data from studies pre-dating or overlapping the early years of ARMOR reflected increasing rates of S. aureus resistance to fluoroquinolones, macrolides, methicillin/oxacillin, and aminoglycosides, while the ARMOR data suggested slight decreases in resistance to these classes between 2009 and 2018. Overall, methicillin-resistant S. aureus (MRSA) prevalence peaked from 2005 to 2015 with a possible decreasing trend in more recent years.
Discussion and Conclusions
Data from local and regional US datasets were generally consistent with data from the national ARMOR surveillance study. Continued surveillance of ocular bacterial pathogens is needed to track trends such as methicillin resistance and MDR prevalence and any new emerging antibiotic resistance phenotypes. Susceptibility data from ARMOR can inform initial choice of therapy, especially in practice areas where local antibiograms are unavailable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In vitro antibiotic susceptibilities for common ocular pathogens from 32 published US studies spanning multiple decades were reviewed and compared against rates from the first 10 years of the ongoing Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) study (2009–2018), the only currently active nationwide surveillance program specific to ocular pathogens. |
Across all studies, high in vitro resistance to fluoroquinolones, macrolides, and methicillin/oxacillin was found among staphylococci, and multidrug resistance was prevalent among methicillin-resistant staphylococci. |
Studies pre-dating or slightly overlapping the early years of the ARMOR study reported increasing rates of in vitro resistance among Staphylococcus aureus to fluoroquinolones, macrolides, methicillin/oxacillin, and aminoglycosides, while the more recent ARMOR data suggested slight decreases in resistance to these classes between 2009 and 2018. |
Other than temporal changes in susceptibility, ARMOR study data were consistent with other locally and regionally reported US susceptibility data validating the use of ARMOR study findings for empiric therapy decision-making in areas with no local antibiograms. |
Introduction
Antibiotic resistance among bacteria is an ongoing concern in all fields of medicine, including ophthalmology. The Centers for Disease Control and Prevention characterizes antibiotic resistance as a leading public health threat and a priority of global significance [1]. While resistance in ocular infections may not be life-threatening, as it may be in other infectious diseases, it can lead to treatment failures that result in morbid consequences, such as blindness or even loss of the affected eye [2,3,4,5,6,7,8,9,10,11,12,13].
Initial choice of therapy for a bacterial ocular infection is almost always made without the benefit of culture and sensitivity results, because cultures are infrequently obtained as part of routine medical care (e.g., conjunctivitis), because of the costs of culturing and sensitivity testing, and/or because results take time, thus potentially delaying treatment in cases where such delays are unacceptable (e.g., keratitis, endophthalmitis). As a result, it is commonplace for treatment to be selected empirically based on knowledge of likely ocular pathogens for the condition being treated and their antibiotic susceptibility profiles. Antibiotic susceptibility data, whether from local/regional studies or from large nationwide surveillance programs, can be an important tool to monitor clinically relevant resistance profiles and track the emergence of any new resistance phenotypes in response to prescribing habits, thus helping guide the selection of initial therapy.
The Ocular Tracking Resistance in US Today (Ocular TRUST) study was the first nationwide surveillance program to track in vitro resistance specifically for bacterial isolates from ocular tissue sources. Ocular TRUST was conducted in the US for only 4 years (2005–2008) [14,15,16]. During the study time frame, the data indicated levels of methicillin resistance among S. aureus and CoNS isolates ranging from 17 to 54% and from 57 to 62%, respectively, as well as multidrug resistance (MDR) to other antibiotic classes.
The Antibiotic Resistance Monitoring in Ocular micRoorganisms (ARMOR) surveillance study was initiated in 2009. It is the only ongoing and currently active nationwide surveillance study in the US specific to common ocular bacterial pathogens. The ARMOR study evaluates clinically relevant isolates of Staphylococcus aureus, coagulase-negative staphylococci (CoNS), Streptococcus pneumoniae, Pseudomonas aeruginosa, and Haemophilus influenzae sourced from any ocular tissue as part of routine medical care. On a yearly basis, predefined numbers of ocular isolates are obtained from community hospitals, academic or university hospitals, specialty or ocular centers, and reference laboratories across the US. Detailed methodology of the ARMOR study and various data analyses at different time points have been published [17,18,19,20,21,22,23,24]. Cumulatively over its first 10 years (2009–2018), the ARMOR study analyzed the in vitro antibiotic susceptibility profiles of > 6000 ocular isolates from throughout the US [25].
Surveillance data, whether local or national, are only useful if considered reliable and if shown to have representative value for a given clinical situation. This review was designed to comprehensively review published ocular pathogen in vitro antibiotic susceptibility data from local and/or regional US studies and compare the study findings against those obtained during the first 10 years of the ARMOR study (2009–2018). The primary goal of this review was to evaluate, at least descriptively, the representativeness of the nationwide ARMOR dataset to the more localized datasets. The analysis also allowed for a broad assessment of ongoing cross-study trends in antibiotic resistance patterns.
Methods
This review article is based on published studies and does not contain any data from new studies with human participants or animals performed by any of the authors.
Search Strategy
MEDLINE®, EMBASE®, and BIOSIS Previews® databases were used to search the titles and abstracts of studies carried out in human subjects and published in English in scholarly journals between January 1, 1995 through June 30, 2021. The following search terms were used with truncations(*) as indicated: (Polybacteria* OR microbial OR bacteria* OR microbiologic* OR etiology OR epidemiology) AND (antibiotic OR fluoroquinolon* OR aminoglycoside* OR antibacterial OR antimicrobial) AND (resistan* OR susceptibility OR susceptible OR sensitivity* OR spectrum OR minimum inhibitory concentration) AND (ophthalm* OR endophthalmitis OR cornea* OR ocular OR keratitis OR conjunctiv* OR intraocular OR blepharitis) AND (infection* OR infectious OR isolat* OR pathogen* OR microorganism*). News reports and case studies were excluded from search results.
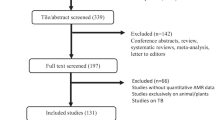
The initial search identified 1109 citations from MEDLINE®, 1688 citations from EMBASE®, and 637 citations from BIOSIS Previews®. Following the removal of duplicates across databases, abstracts, and full papers when necessary, were then reviewed to exclude publications with any of the following characteristics: non-US studies; studies focused solely on bacterial organisms and/or antibiotics not included in ARMOR; studies without organism-specific susceptibility data; studies focused on non-clinically relevant (non-pathogenic) bacterial organisms (e.g., normal flora); studies that did not have a minimum of 20 isolates for at least one bacterial species included in ARMOR; studies that separated data by, or limited data to, unique patient or organism characteristics or situations (e.g., prior use of antibiotics or purposeful selection of organisms with certain resistance characteristics such as fluoroquinolone or vancomycin resistance); studies in which more than half of the data collection years preceded 1995; review papers; and ARMOR-related datasets that were published prior to the 10-year analysis [25]. Reference lists in relevant publications were reviewed to identify additional articles for inclusion.
Data Analysis
Published surveillance data from local/regional studies were compared descriptively against corresponding data from the 10-year ARMOR dataset. The primary focus was cross-study antibiotic susceptibility patterns for S. aureus, CoNS, S. pneumoniae, P. aeruginosa, and H. influenzae isolates, the bacterial pathogens that are specifically collected in ARMOR. Wherever possible, cross-study susceptibility data were compared within categories of the same ocular tissue or clinical diagnosis corresponding to that tissue (e.g., conjunctivitis/conjunctiva; keratitis/cornea; endophthalmitis/intraocular [aqueous or vitreous humor]).
Secondary cross-study analyses included MDR among staphylococci, longitudinal changes in susceptibility over time, and minimum inhibitory concentration (MIC) data. Other than in vitro antibiotic susceptibility data, which were a prerequisite for study inclusion, not every study reported data relevant for all secondary analyses.
Antibiotic susceptibility analyses were limited to drugs and classes of drugs assessed in ARMOR, including fluoroquinolones, macrolides, chloramphenicol, the beta-lactam oxacillin, tetracycline, aminoglycosides, trimethoprim, and vancomycin. For studies reporting susceptibility data for multiple drugs per class, the default drug chosen was aligned with that used in the ARMOR study to define class resistance, namely: ciprofloxacin for fluoroquinolone resistance (moxifloxacin if ciprofloxacin was not reported); azithromycin for macrolide resistance (erythromycin if azithromycin was not reported); and tobramycin for aminoglycoside resistance. In the analysis of all published data, including ARMOR, methicillin resistance was defined by resistance to oxacillin or methicillin. Multidrug resistance was defined as resistance to at least one antimicrobial agent in at least three or more drug classes.
Results
Antibiotic Class Susceptibility
The literature search identified 32 studies with relevant in vitro antibiotic susceptibility data that could be compared to data from the ARMOR study [8, 14,15,16, 26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. Figure 1 illustrates antibiotic class in vitro susceptibilities by ocular pathogen and diagnosis/ocular tissue source, where available, for these US studies and the ARMOR study. Numerical values for each data point can be found in Table S1 in the Supplemental Material. Note that in the 10-year ARMOR dataset, the ocular tissue source (conjunctiva, cornea, aqueous humor/vitreous humor) was known for 51% of isolates. Thus, Fig. 1 presents ARMOR data both for all isolates overall regardless of tissue source and by ocular tissue source where known. Also, unless stated otherwise, ARMOR data cited in the following paragraphs reflect that for the overall ARMOR dataset (i.e., regardless of diagnosis/tissue source).
Antibiotic class in vitro susceptibility of common ocular bacterial pathogens (US studies). Data points represent the percentages of pathogens susceptible to the antibiotic classes indicated along the bottom of the figure. Where reported as such, data are presented by ocular diagnosis/tissue source (top labels C, K, E; see explanatory legend below panel A). Data without a known ocular diagnosis/tissue source and/or data inclusive of multiple diagnoses/tissue sources are depicted by horizontal lines spanning the antibiotic category. Red squares/lines represent ARMOR dataa; black circles/lines represent other published data with time frames at least partially contemporary with ARMOR (2009–2018); gray circles/lines represent other published data with time frames exclusively older than ARMOR (pre-2009). For studies reporting resistance rates by individual year only, most recent year data are reflected. Only studies with pathogen samples consisting of ≥ 20 isolates per species are included. Source data can be viewed in Table S1 of the Supplemental Material. AG aminoglycosides, CHL chloramphenicol, CoNS coagulase-negative staphylococci, FQ fluoroquinolones, MET methicillin/oxacillin, ML macrolides, MRSA methicillin-resistant Staphylococcus aureus, MSSA methicillin-susceptible Staphylococcus aureus, PEN penicillin, TET tetracycline, VAN vancomycin. aNote: For the ARMOR study, the horizontal data lines reflect all ARMOR data for that pathogen/antibiotic class combination and include the tissue source-specific data represented by the red square plot points in the same categories. Tissue source was unknown for about half (49%) of all isolates collected in ARMOR. A S. aureus. B MSSA. Note: Markers labeled “X2” indicate the presence of 2 data points with identical values at the indicated plot point. C MRSA. Note: Markers labeled “X2” indicate the presence of 2 data points with identical values at the indicated plot point. D CoNS. S. epi = Staphylococcus epidermidis. E S. pneumoniae. F P. aeruginosa. Note: Markers labeled “X2” or “X3” denote the presence of 2 or 3 data points, respectively, with identical values at the indicated plot point. G H. influenzae
In the ARMOR dataset, 66.5% of S. aureus isolates were susceptible to fluoroquinolones; other studies reported percentages ranging from 34.7 to 81.8% (Fig. 1A). For ARMOR isolates with known tissue source, susceptibility to these antibiotics varied by tissue source: endophthalmitis (57.1%), keratitis (64.3%), and conjunctivitis (71.6%). In ARMOR, 40.3% of S. aureus isolates overall were susceptible to macrolides, with other studies ranging from 25 to 62.1%. As for methicillin resistance, 65.1% of S. aureus isolates in ARMOR were susceptible to oxacillin, while other studies ranged from 45.0 to 76%. Methicillin-resistant S. aureus (MRSA) rates tended to be higher among ARMOR isolates from endophthalmitis (46.9%) and keratitis (34.3%) compared to conjunctivitis (28.9%). ARMOR and other studies consistently indicated high susceptibility of S. aureus to chloramphenicol (ARMOR, 94.3%; others, 86.4%), tetracycline (ARMOR, 93.9%; others, 78.0–84.0%), aminoglycosides (ARMOR, 84.4%; others, 75.0–95.0%), trimethoprim (ARMOR, 95.7%; others, 95.7%), and vancomycin (ARMOR, 100%; others, 99–100%).
Susceptibility data for methicillin-susceptible S. aureus (MSSA) isolates (Fig. 1B) showed a high degree of cross-study similarity with high susceptibility to most antibiotics reported, including fluoroquinolones (ARMOR, 88.6%; others, 79.9–96.0%), tetracycline (ARMOR, 97.1%; others, 78.6–94.1%), aminoglycosides (ARMOR, 96.7%; others, 91.9–100%), trimethoprim (ARMOR, 96.3%; others, 96.0–100%), and vancomycin (ARMOR, 100%; others, 99.0–100%). For the macrolide class, MSSA isolates demonstrated relatively lower susceptibility percentages (ARMOR, 58.2%; others, 52.0–72.0%).
Data for MRSA isolates showed low susceptibility to fluoroquinolones (ARMOR, 25.5%; others, 4.3–55.0%) and macrolides (ARMOR, 7.1%; others, 0–10.7%) across studies (Fig. 1C). Conversely, susceptibilities were uniformly high for chloramphenicol (ARMOR, 90.4%; others, 90.0–90.5%), tetracycline (ARMOR, 85.0%; others, 87.2–100%), trimethoprim (ARMOR, 94.5%; others, 70—96.1%), and vancomycin (ARMOR, 100%; others, 99.0–100%). Susceptibility data for MRSA isolates to aminoglycosides showed a high degree of variability among studies, with ARMOR data being approximately mid-range between susceptibility data points from other studies (ARMOR 61.6%; others, 36.4—95.0%).
For CoNS isolates, the collective data indicated moderate susceptibility to fluoroquinolones (ARMOR, 65.8%; others, 44–93.0%), macrolides (ARMOR, 38.6%; others, 34.3–52%), and methicillin/oxacillin (ARMOR, 50.7%; others, 45–70.4%) (Fig. 1D). In the ARMOR study, susceptibility to fluoroquinolones appeared to be impacted by tissue source, with endophthalmitis isolates (47.8%) showing lower susceptibility to these antibiotics compared to keratitis (63.9%) or conjunctivitis (70.8%) isolates. Overall, susceptibility was high to chloramphenicol (ARMOR, 98.8%; others, 70–95.5%), tetracycline (ARMOR, 87.6%; others, 75.7–90.9%), aminoglycosides (ARMOR, 82.5%; others, 72–95.3%), and vancomycin (ARMOR, 100%; others, 99–100%). In two non-ARMOR studies, susceptibility rates of Staphylococcus epidermidis endophthalmitis isolates were notably lower than those for non-S. epidermidis CoNS isolates for fluoroquinolones and aminoglycosides (Fig. 1D).
Data for S. pneumoniae isolates suggested a high degree of susceptibility to fluoroquinolones (ARMOR, 99.8%; others, 85.0–100%), chloramphenicol (ARMOR, 96.9%; others, 96.4–97.1%), and tetracycline (ARMOR, 91.3%; others, 86.4–90.0%), while macrolides (ARMOR, 63.7%; others, 59.0–95.0%) and penicillin (ARMOR, 67.8%; others, 51.4–88%) were characterized by moderate to high susceptibility and less congruence between studies (Fig. 1E). For the macrolides and penicillin, lower susceptibility percentages were noted among published studies at least partially contemporary with ARMOR compared to those with exclusively pre-2009 data.
For P. aeruginosa isolates, published susceptibility percentages for fluoroquinolones (ARMOR, 92.8%; others, 95.0–100%) and aminoglycosides (ARMOR, 97.1%; others, 94–100%) were high and consistent between studies (Fig. 1F).
Published data for H. influenzae isolates showed uniformly high susceptibility, with almost all studies reporting close to 100% susceptibility to fluoroquinolones, macrolides, and chloramphenicol (Fig. 1G).
Multidrug Resistance
Including the ARMOR study, only five studies reported data pertaining to the prevalence of MDR. Three studies reported rates of MDR among MRSA isolates within overlapping time frames, ranging from 42.9% (2010–2015 data from New York) [44] to 91.5% (2006–2016 data from Florida) [46] compared with 75.4% reported in the ARMOR study [25]. Furthermore, while a specific percentage was not cited, Asbell et al. reported that “MRSA was resistant to almost every agent, including the newer fluoroquinolones” in The Surveillance Network nationwide study conducted in the US from 2000 to 2005 [27]. The ARMOR study (2009–2018) noted MDR in 41.2% of all CoNS isolates and 73.7% of all methicillin-resistant CoNS (MRCoNS) isolates nationwide [25]. Schechter et al. [48] reported that 30% of S. epidermidis isolates and 75% of methicillin-resistant S. epidermidis (MRSE) isolates were multidrug-resistant (2017 data).
Longitudinal Changes in Antibiotic Susceptibilities
Including the ARMOR study, nine studies reported statistically significant longitudinal changes in antibiotic susceptibilities for specific pathogens (Table 1). For S. aureus, earlier studies suggested increasing resistance to multiple classes of antibiotics over time, including fluoroquinolones, macrolides, the beta-lactams methicillin/oxacillin, and aminoglycosides, while the ARMOR 2009–2018 study found decreasing resistance to each of these antibiotic classes [25]. Marangon et al. [39] reported an increase in resistance to fluoroquinolones from 7.5 to 39.6% between 1990–2001. An increase in resistance of S. aureus isolates to fluoroquinolones from 6 to 36% was observed between 1997 and 2008 (change of 2.57% per year) by Adebayo et al. [26]. In the ARMOR study, fluoroquinolone resistance among S. aureus isolates decreased 2.24% per year between 2009 (38.5%) and 2018 (30.0%) [25]. Macrolide resistance among S. aureus isolates increased 3.74% per year between 1997 (~ 20%) and 2008 (~ 75%) in one study [26] but was shown to decrease 1.4% per year between 2009 (61.5%) and 2018 (56.3%) in the ARMOR dataset [25].
Increases in S. aureus resistance to beta-lactam antibiotics (generally oxacillin) were reported in the time periods from 1993–1996 (18.4%) to 2009–2012 (38.3%) (+ 19.9%) [29], from 1992–1996 (23%) to 2007–2011 (55%) (+ 32%) [32], from 2000 (29.5%) to 2005 (41.6%) (+ 12.1%) [27], and from 1997 (~ 2%) to 2008 (~ 40%) (+ 3.69% per year) [26]. In the ARMOR study, oxacillin resistance among S. aureus isolates decreased by 2.16% per year between 2009 (39.0%) and 2018 (29.3%) [25]. Figure 2 presents data points pertaining to reported prevalence of MRSA (resistance to oxacillin or methicillin) as a proportion of S. aureus isolates by year. Overall, reported prevalence was highest during the periods from 2005 to 2015 and shows some signs of decreasing in most recent years.
Aminoglycoside resistance among S. aureus isolates increased by 0.36% per year from 1997 (~ 7%) to 2008 (~ 10%) as reported by Adebayo et al. [26] but decreased by 1.84% per year from 2009 (23.5%) to 2018 (10.7%) in the ARMOR study [25].
For MRSA isolates, older studies reported trends of increasing resistance among MRSA to fluoroquinolones, including a 27.9% increase between 1990 (55.8%) and 2001 (83.7%) [39] and an increase from approximately 10% during 1993–1996 to approximately 45% during 2009–2012 [29]; the ARMOR study noted no significant changes among MRSA in resistance rates to fluoroquinolones, macrolides, chloramphenicol, or tetracycline between 2009 and 2018, and found decreasing resistance in MRSA isolates to aminoglycosides between 2009 and 2018 [25].
For CoNS isolates, one study reported a 28% increase in CoNS resistance to fluoroquinolones between the time periods 1995–1999 (28%) to 2010–2016 (56%) [51]. In addition, data from the Bascom Palmer Eye Institute reported CoNS resistance to ciprofloxacin increased from 10.3% during the period 1990–1994 to 60.5% during the period 2005–2011 [49, 54] (no statistics provided, thus not included in Table 1). In contrast, the ARMOR study revealed decreasing resistance to fluoroquinolones by a rate of –1.38% per year between 2009 (45.8%) and 2018 (33.6%) [25]. The ARMOR study found no significant changes in CoNS resistance to oxacillin from 2009 to 2018 but did indicate a slight increase in resistance to aminoglycosides of + 0.71% per year [25]. There were no other published longitudinal findings for CoNS except a small study by Gentile et al. [32], which reported a 24% increase in S. epidermidis resistance to oxacillin between the periods 1987–1991 (31% resistant) and 2007–2011 (55% resistant).
For P. aeruginosa, S. pneumoniae, and H. influenzae isolates, the ARMOR 10-year data did not demonstrate any significant changes in resistance to any of the antibiotic classes tested. In one other published study that reported longitudinal data for these organisms between 1997 and 2008 [26], small increases were noted for S. pneumoniae resistance to macrolides (1997, ~ 1%; 2008, ~ 5%; + 0.38% per year) and tetracycline (1997, ~ 1%; 2008, ~ 10%; + 0.85% per year) and for H. influenzae resistance to tetracycline (1997, ~ 3%; 2008, ~ 25%; + 2.18% per year).
Minimum Inhibitory Concentrations (MICs)
Table S2 in the Supplemental Material provides MIC data that were presented in several of the studies [14, 25, 33,34,35, 38, 44]. Among staphylococcal isolates, where reported, vancomycin MICs were consistently low whereas macrolide MICs were consistently high. A comparison of susceptibility rates for individual fluoroquinolones reported in various published studies did not reveal many differences between individual agents (ciprofloxacin, levofloxacin, gatifloxacin, moxifloxacin; Fig. S1 in the Supplemental Material), even though reported MICs varied between them, particularly for staphylococcal isolates, with 8-methoxy fluoroquinolones demonstrating better in vitro potency in general (Table S2 in the Supplemental Material).
Discussion
The ARMOR study is the only currently active nationwide surveillance program that monitors in vitro bacterial resistance to antibiotics among common ocular pathogens. Given the importance of reliable surveillance data for guiding empiric therapy in ocular infections, the representativeness of the ARMOR data is an important consideration. In this analysis, antibiotic susceptibility data specific to ocular bacterial pathogens from US studies published in the past 25 years were compared against susceptibility data from the first ten years of the ARMOR program. Of the 32 comparative reports included in this review, all were single-center and/or regional US studies except one that was also comprised of national data, namely the Ocular TRUST study [14,15,16] but was conducted in the years prior to initiation of ARMOR.
Across all studies, high levels of in vitro resistance were found among S. aureus and CoNS to fluoroquinolones, macrolides, and methicillin/oxacillin. As expected, MSSA isolates showed high susceptibility to all antibiotic classes evaluated in all studies, except for moderate susceptibility to macrolides, while MRSA isolates were shown to have generally low susceptibility to fluoroquinolones and macrolides. In contrast to findings for staphylococci, high levels of susceptibility were observed across studies to fluoroquinolones, chloramphenicol, and tetracycline among S. pneumoniae, to fluoroquinolones and aminoglycosides among P. aeruginosa, and to fluoroquinolones, macrolides, and chloramphenicol among H. influenzae. Overall, ARMOR 10-year cumulative susceptibility data were generally mid-range of, or similar to, other published US susceptibility data for common ocular pathogens. Furthermore, there was generally a high degree of concordance among resistance rates across studies by ocular diagnoses/tissue sources (conjunctivitis/conjunctiva, keratitis/cornea, endophthalmitis/aqueous or vitreous humor), with few exceptions. For instance, in the ARMOR study, MSSA intraocular isolates (n = 26) had greater susceptibility to macrolides compared to most other studies and compared to isolates from other ocular sources in ARMOR; however, the isolates in this subset only constitute about 2% of the total ARMOR MSSA dataset. Additionally, among S. aureus and CoNS, there was an observed trend for lower susceptibility to fluoroquinolones among endophthalmitis/intraocular-sourced isolates than among those from other diagnoses/ocular sources. These findings may reflect greater exposure of staphylococcal intraocular isolates to the fluoroquinolone class as these antibiotics are widely used for prophylaxis of endophthalmitis, either as topical eyedrops applied before and after an intraocular procedure or as intracameral injections in the cataract surgery setting [55].
Multidrug resistance can pose a major obstacle to effective treatment, yet few of the studies reviewed reported on this phenomenon. Methicillin resistance is often a hallmark of resistance to other antibiotic classes [14, 29], and indeed three out of four MRSA and MRCoNS isolates from the ARMOR study exhibited MDR. Other relatively recent data reviewed here also reported high rates of MDR among MRSA [44, 46] and MRSE [48]. Together, these data underscore the potential challenges to the management of ocular infections caused by methicillin-resistant staphylococci, as these organisms are highly resistant to the first line of antibiotics commonly used in ophthalmology. This adds to the importance of the availability of reliable and contemporary susceptibility data. On the other hand, MRSA isolates were shown in most studies to have reasonably high rates of susceptibility to chloramphenicol, tetracycline, trimethoprim, and vancomycin. One may expect similarly high susceptibilities to these drugs among the subset of MRCoNS isolates; however, there were insufficient studies with data on this resistance phenotype group for comparison.
For S. aureus, the collective data from all studies suggested increasing in vitro resistance to multiple antibiotic classes over time in the 1990s and 2000s. Studies reporting data from ocular isolates collected more recently suggested a plateau and even decreasing resistance among S. aureus since then, most notably for methicillin/oxacillin, a finding in line with the decreasing incidence of systemic MRSA infections observed in recent years by the Center for Disease Control and a similar decreasing MRSA rate in the global SENTRY surveillance study [56, 57]. The ARMOR study, which provides the most recent data (2009–2018) of the published studies, demonstrated small but statistically significant decreases in antibiotic resistance of S. aureus to fluoroquinolones, oxacillin, macrolides, chloramphenicol, and aminoglycosides. With the exception of chloramphenicol, these decreasing trends appear to have persisted through 2019 as reported in an interim analysis of ARMOR data [58]. Furthermore, for MRSA isolates, studies with datasets reflecting the time periods from 1990–2001 [39] and 1993–2012 [29] noted significant increases in resistance to fluoroquinolones, whereas ARMOR study isolates collected from 2009–2018 did not demonstrate any significant changes in MRSA resistance to fluoroquinolones over that time frame, which suggests that such resistance may have stabilized during those years. For CoNS, one study reported a 28% increase in CoNS resistance to fluoroquinolones from 1995 to 2016 [51], whereas the ARMOR study demonstrated a small but significant yearly decrease in CoNS resistance to fluoroquinolones for the more recent time period of 2009 to 2018 [25]. Furthermore, a 24% increase in S. epidermidis resistance to oxacillin was noted in one study between the time periods 1987–1991 and 2007–2011 [32], while the more recent ARMOR study found no significant change in CoNS resistance to methicillin/oxacillin between 2009 and 2018. While the comparator studies that included organisms collected prior to or in the early years of the ARMOR program found increasing rates of resistance to clinically relevant antibiotics, ARMOR findings suggest potential recent reductions (or stabilization) in antibiotic resistance among staphylococci. It is important to note that most of the comparator studies reported rates of resistance, especially to topical fluoroquinolones, in subsets of isolates recovered from specific groups of diseases where these antibiotics are widely used empirically for treatment (e.g., keratitis) or prophylaxis (e.g., endophthalmitis), while the ARMOR data on resistance trends over time were not stratified by infection type to allow evaluation of any potential disease/tissue-specific trends in the population analyzed. If the slight decreases in resistance suggested by the more recent ARMOR data are true, these positive trends might reflect improved antibiotic stewardship in clinical practice, but clearly further study is necessary to confirm these trends.
Minimum inhibitory concentrations for a given ocular isolate can be useful for comparing relative in vitro potency of specific agents, particularly for different compounds within the same drug class. Studies have suggested a correlation between MICs and clinical outcomes in patients with corneal infections [13, 59, 60], indicating that MIC data that have not been interpreted further (as susceptible, intermediate, or resistant) could also have value in choosing empiric treatment. A comparison of available MIC data in reviewed studies found that fluoroquinolone agents had similar in vitro potency against gram-negative organisms, although the newer-generation 8-methoxy fluoroquinolones (moxifloxacin and gatifloxacin) generally exhibited higher in vitro potency (lower MICs) compared to older fluoroquinolones (ciprofloxacin and levofloxacin) against gram-positive isolates, especially staphylococci. While only evaluated in ARMOR and two other studies reviewed herein, MICs for besifloxacin, an 8-chloro-fluoroquinolone FDA-approved in 2009, were typically among the lowest of the fluoroquinolones. In the 10-year ARMOR dataset, besifloxacin MICs that inhibited growth of 90% of isolates in the studied population (MIC90s), were at least fourfold lower than moxifloxacin and gatifloxacin and at least 16-fold lower than ciprofloxacin and levofloxacin for gram-positive bacteria including S. aureus, CoNS, and S. pneumoniae [25]. Marketed exclusively as an ocular formulation, besifloxacin has no CLSI systemic breakpoints and was therefore not included in any susceptibility assessments in the studies reviewed here.
Although not the primary focus of this review, pathogen distributions by diagnosis/ocular tissue in identified studies were examined (Table S3 in the Supplemental Material). While distributions by diagnoses/ocular tissues were observed to differ across studies, S. aureus and CoNS/S. epidermidis were cited as “prevalent” (one of the top five most prevalent pathogens) in all diagnoses (conjunctivitis, keratitis, and endophthalmitis) in almost every study with relevant data. As endophthalmitis often results from introduction of organisms originating from the ocular microbiota secondary to penetration of the ocular surface through surgery or trauma [61, 62], a high rate of infection with these organisms is not surprising. Haemophilus influenzae was prevalent in conjunctivitis sources but not in keratitis or endophthalmitis cases. Pseudomonas aeruginosa was consistently reported as prevalent in keratitis; it was noted as prevalent in conjunctivitis in fewer than half of relevant studies and in none of the studies with endophthalmitis data. Thus, data from the published studies with prevalence data confirm that the bacterial pathogens selected for study in ARMOR (S. aureus, CoNS, S. pneumoniae, P. aeruginosa, and H. influenzae) do appear to be among the most common species isolated from various eye infections in US patients. Also, although a formal analysis was not conducted, there were no apparent differences in pathogen distribution by geography.
Among the limitations of this review is that the studies evaluated were not uniform with regard to antibiotics tested, bacterial pathogens included, and ocular diagnoses. Data included were limited to the bacterial pathogens and antibiotics tested in ARMOR, thereby excluding information on other, albeit less common, bacterial species involved in ocular infections. There were also differences in methodologies used to determine resistance profiles (e.g., MIC vs. E-test vs. disk diffusion), which could introduce slight variations in data between studies. CLSI breakpoints are occasionally updated, allowing for the possibility that isolates categorized at one time point as susceptible might have been categorized as non-susceptible at a different time point, or vice versa. As well, patient age-associated differences in ocular pathogen distributions and antibiotic susceptibilities have been observed in ARMOR analyses and other studies [17, 18, 22, 44, 63,64,65,66]; however, very few of the studies included in this review provided patient age data, thus precluding cross-study comparisons adjusting for this confounding factor and likely contributing to some of the between-study variabilities observed. Yet, it could be argued that the similarities found among much of the findings, regardless of cross-study methodology differences and control for confounding variables, add to the strength of the observations. Finally, this analysis evaluated in vitro data only, and almost none of the studies included information on clinical outcomes of microbial eradication or clinical resolution. For ocular infections, susceptibility classifications should be interpreted with an understanding of how they are determined. CLSI breakpoints used for susceptibility determinations are based on pharmacokinetics and pharmacodynamics of antibiotics when administered systemically. As there are no specific ocular breakpoints for susceptibility, systemic breakpoints are currently all that are available for assessing in vitro susceptibility even though topical ocular administration of medication is a distinct and very different milieu. Antibiotics administered topically to the eye would be expected to achieve much higher initial concentrations than concentrations achieved in the blood with systemic administration, but factors such as tear dilution and elimination from the eye can lower concentrations on the eye very rapidly. Accordingly, the clinical relevance of in vitro susceptibility data for ocular infections is unclear.
Conclusions
Overall, this review of published studies featuring in vitro susceptibility data for common ocular bacterial pathogens found high levels of in vitro resistance to fluoroquinolones, macrolides, and methicillin/oxacillin among staphylococci, as well as prevalent MDR in these pathogens, particularly among methicillin-resistant staphylococci. The collective data reviewed herein also reveal a longitudinal pattern of increasing in vitro resistance rates for ocular staphylococci to multiple classes of antibiotics over past decades, but that this trend may be potentially showing signs of reversing or stabilizing. Overall findings suggest that the ongoing national ARMOR study reports resistance data that are generally consistent with that from other studies reporting local and regional US data. As such, it lends support to the reliability of the ARMOR findings for identifying trends in susceptibility and its clinical usefulness in informing empiric therapy decision-making, especially where local and/or current antibiograms are not available. Continued monitoring of antibiotic susceptibility data in ocular bacteria is critical to track these trends and maintain vigilance for the emergence of important resistance phenotypes.
References
Centers for Disease Control and Prevention. Antibiotic resistance: a global threat. 2020. https://www.cdc.gov/drugresistance/solutions-initiative/stories/ar-global-threat.html. Accessed 11 May 2021.
Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79(3):214–21.
Song X, Xie L, Tan X, et al. A multi-center, cross-sectional study on the burden of infectious keratitis in China. PLoS ONE. 2014;9(12): e113843. https://doi.org/10.1371/journal.pone.0113843.
Lin A, Rhee MK, Akpek EK, et al. for the Cornea/External Disease Preferred Practice Panel 2017–2018. Bacterial Keratitis Preferred Practice Pattern®. American Academy of Ophthalmology; 2018. https://doi.org/10.1016/j.ophtha.2018.10.018.
Jin H, Parker WT, Law NW, et al. Evolving risk factors and antibiotic sensitivity patterns for microbial keratitis at a large county hospital. Br J Ophthalmol. 2017;101(11):1483–7. https://doi.org/10.1136/bjophthalmol-2016-310026.
Novosad BD, Callegan MC. Severe bacterila endophthalmitis: towards improving clinical outcomes. Expert Rev Ophthalmol. 2010;5(5):689–98. https://doi.org/10.1586/eop.10.52.
Lu X, Ng DS-C, Zheng K, et al. Risk factors for endophthalmitis requiring evisceration or enucleation. Sci Rep. 2016;6:8100. https://doi.org/10.1038/srep28100.
Amato M, Pershing S, Walvick M, Tanaka S. Trends in ophthalmic manifestations of methicillin-resistant Staphylococcus aureus (MRSA) in a northern California pediatric population. J AAPOS. 2013;17(3):243–7. https://doi.org/10.1016/j.jaapos.2012.12.151.
Chatterjee S, Agrawal D. Multi-drug resistant Pseudomonas aeruginosa keratitis and its effective treatment with topical colistimethate. Indian J Ophthalmol. 2016;64(2):153–7. https://doi.org/10.4103/0301-4738.179721.
Garg P, Sharma S, Rao GN. Ciprofloxacin-resistant Pseudomonas keratitis. Ophthalmology. 1999;106(7):1319–23. https://doi.org/10.1016/S0161-6420(99)00717-4.
Moshirfar M, Mirzaian G, Feiz V, Kang PC. Fourth-generation fluoroquinolone-resistant bacterial keratitis after refractive surgery. J Cataract Refract Surg. 2006;32(3):515–8. https://doi.org/10.1016/j.jcrs.2005.12.108.
Segreti J, Jones RN, Bertino JS Jr. Challenges in assessing microbial susceptibility and predicting clinical response to newer-generation fluoroquinolones. J Ocul Pharmacol Ther. 2012;28(1):3–11. https://doi.org/10.1089/jop.2011.0072.
Wilhelmus KR, Abshire RL, Schlech BA. Influence of fluoroquinolone susceptibility on the therapeutic response of fluoroquinolone-treated bacterial keratitis. Arch Ophthalmol. 2003;121(9):1229–33. https://doi.org/10.1001/archopht.121.9.1229.
Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: Nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol. 2008;145:951–8. https://doi.org/10.1016/j.ajo.2008.01.025.
Asbell PA, Sahm DF. Longitudinal nationwide antimicrobial susceptibility surveillance in ocular isolates: results from Ocular TRUST 2. Presented at: American Society of Cataract and Refractive Surgery Annual Meeting; April 28, 2008; San Diego, CA.
Asbell PA, Sahm DF, Shedden A. Ocular TRUST 3: ongoing longitudinal surveillance of antimicrobial susceptibility in ocular isolates. Presented at: American Society of Cataract and Refractive Surgery Annual Meeting; April 7, 2009; San Francisco, CA.
Thomas RK, Melton R, Asbell PA. Antibiotic resistance among ocular pathogens: current trends from the ARMOR Surveillance Study (2009–2016). Clin Optom (Auckl). 2019;11:15–26. https://doi.org/10.2147/OPTO.S189115.
Asbell PA, DeCory HH. Antibiotic resistance among bacterial conjunctival pathogens collected in the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) Surveillance Study. PLoS ONE. 2018;13(10): e0205814. https://doi.org/10.1371/journal.pone.0205814.
Alter SJ, Sanfilippo CM, Asbell PA, DeCory HH. Antibiotic resistance among pediatric-sourced ocular pathogens: 8-year findings from the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) Surveillance Study. Pediatr Infect Dis J. 2019;38(2):138–45. https://doi.org/10.1097/INF.0000000000002206.
Asbell PA, Pandit RT, Sanfilippo CM. Antibiotic resistance rates by geographic region among ocular pathogens collected during the ARMOR surveillance study. Ophthalmol Ther. 2018;7(2):417–29. https://doi.org/10.1007/s40123-018-0141-y.
Asbell PA, Mah FS, Sanfilippo CM, DeCory HH. Antibiotic susceptibility of bacterial pathogens isolated from the aqueous and vitreous humor in the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) Surveillance Study. J Cataract Refract Surg. 2016;42(12):1841–3. https://doi.org/10.1016/j.jcrs.2016.11.008.
Asbell PA, Sanfilippo CM, Pillar CM, DeCory HH, Sahm DF, Morris TW. Antibiotic resistance among ocular pathogens in the United States: five-year results from the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) Surveillance Study. JAMA Ophthalmol. 2015;133(12):1445–54. https://doi.org/10.1001/jamaophthalmol.2015.3888.
Haas W, Pillar CM, Torres M, Morris TW, Sahm DF. Monitoring antibiotic resistance in ocular microorganisms: results from the Antibiotic Resistance Monitoring in Ocular micRorganisms (ARMOR) 2009 Surveillance Study. Am J Ophthalmol. 2011;152(4):567-574.e3. https://doi.org/10.1016/j.ajo.2011.03.010.
Asbell PA, Sanfilippo CM. Antibiotic resistance trends among ocular pathogens in the US—cumulative results from the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) surveillance study. US Ophthalmic Rev. 2017;10(1):35–8. https://doi.org/10.17925/USOR.2017.10.01.35.
Asbell PA, Sanfilippo CM, Sahm DF, DeCory HH. Trends in antibiotic resistance among ocular microorganisms in the United States From 2009 to 2018. JAMA Ophthalmol. 2020;138(5):439–50. https://doi.org/10.1001/jamaophthalmol.2020 (eAppendix Web-based Interactive Data Visualization Tool accessed April 14, 2021).
Adebayo A, Parikh JG, McCormick SA, et al. Shifting trends in in vitro antibiotic susceptibilities for common bacterial conjunctival isolates in the last decade at the New York Eye and Ear Infirmary. Graefes Arch Clin Exp Ophthalmol. 2011;249(1):111–9. https://doi.org/10.1007/s00417-010-1426-6.
Asbell PA, Sahm DF, Shaw M, Draghi DC, Brown NP. Increasing prevalence of methicillin resistance in serious ocular infections caused by Staphylococcus aureus in the United States: 2000 to 2005. J Cataract Refract Surg. 2008;34(5):814–8. https://doi.org/10.1016/j.jcrs.2008.01.016.
Benz MS, Scott IU, Flynn HW Jr, Unonius N, Miller D. Endophthalmitis isolates and antibiotic sensitivities: a 6-year review of culture-proven cases. Am J Ophthalmol. 2004;137(1):38–42. https://doi.org/10.1016/s0002-9394(03)00896-1.
Chang VS, Dhaliwal DK, Raju L, Kowalski RP. Antibiotic resistance in the treatment of Staphylococcus aureus Keratitis: a 20-year review. Cornea. 2015;34(6):698–703. https://doi.org/10.1097/ICO.0000000000000431.
Elsahn AF, Yildiz EH, Jungkind DL, et al. In vitro susceptibility patterns of methicillin-resistant Staphylococcus aureus and coagulase-negative Staphylococcus corneal isolates to antibiotics. Cornea. 2010;29(10):11331–21135. https://doi.org/10.1097/ICO.0b013e3181d2ce25 (Erratum. in: Cornea. 2010;29(12):1470).
Geevarghese A, Shah P, Lopez J, Tsui E, Raju L. Common microbes and antibiotics in ocular infections at an urban public tertiary care hospital. Ocul Immunol Inflamm. 2020. https://doi.org/10.1080/09273948.2020.1799033.
Gentile RC, Shukla S, Shah M, et al. Microbiological spectrum and antibiotic sensitivity in endophthalmitis: a 25-year review. Ophthalmology. 2014;121(8):1634–42. https://doi.org/10.1016/j.ophtha.2014.02.001.
Haas W, Gearinger LS, Hesje CK, Sanfilippo CM, Morris TW. Microbiological etiology and susceptibility of bacterial conjunctivitis isolates from clinical trials with ophthalmic, twice-daily besifloxacin. Adv Ther. 2012;29(5):442–55. https://doi.org/10.1007/s12325-012-0023-y.
Haas W, Hesje CK, Sanfilippo CM, Morris TW. High proportion of nontypeable Streptococcus pneumoniae isolates among sporadic, nonoutbreak cases of bacterial conjunctivitis. Curr Eye Res. 2011;36(12):1078–85. https://doi.org/10.3109/02713683.2011.624670.
Harper T, Miller D, Flynn HW Jr. In vitro efficacy and pharmacodynamic indices for antibiotics against coagulase-negative Staphylococcus endophthalmitis isolates. Ophthalmology. 2007;114(5):871–5. https://doi.org/10.1016/j.ophtha.2007.01.007.
Hsu HY, Ernst B, Schmidt EJ, Parihar R, Horwood C, Edelstein SL. Laboratory results, epidemiologic features, and outcome analyses of microbial keratitis: A 15-year review from St. Louis. Am J Ophthalmol. 2019;198:54–62. https://doi.org/10.1016/j.ajo.2018.09.032.
Kodati S, Eller AW, Kowalski RP. The susceptibility of bacterial endophthalmitis isolates to vancomycin, ceftazidime, and amikacin: a 23 year-review. Ophthalmol Retina. 2017;1(3):206–9. https://doi.org/10.1016/j.oret.2016.11.010.
Kowalski RP, Dhaliwal DK, Karenchak LM, et al. Gatifloxacin and moxifloxacin: an in vitro susceptibility comparison to levofloxacin, ciprofloxacin, and ofloxacin using bacterial keratitis isolates. Am J Ophthalmol. 2003;136(3):500–5. https://doi.org/10.1016/s0002-9394(03)00294-0.
Marangon FB, Miller D, Muallem MS, Romano AC, Alfonso EC. Ciprofloxacin and levofloxacin resistance among methicillin-sensitive Staphylococcus aureus isolates from keratitis and conjunctivitis. Am J Ophthalmol. 2004;137(3):4534–58. https://doi.org/10.1016/j.ajo.2003.10.026.
Miller D. Update on the epidemiology and antibiotic resistance of ocular infections. Middle East Afr J Ophthalmol. 2017;24(1):30–42. https://doi.org/10.4103/meajo.MEAJO_276_16.
Miller JJ, Scott IU, Flynn HW Jr, Smiddy WE, Corey RP, Miller D. Endophthalmitis caused by Streptococcus pneumoniae. Am J Ophthalmol. 2004;138(2):231–6. https://doi.org/10.1016/j.ajo.2004.03.008.
Ni N, Nam EM, Hammersmith KM, et al. Seasonal, geographic, and antimicrobial resistance patterns in microbial keratitis: 4-year experience in eastern Pennsylvania. Cornea. 2015;34(3):296–302. https://doi.org/10.1097/ICO.0000000000000352.
Ohnsman C, Ritterband D, O’Brien T, Girgis D, Kabat A. Comparison of azithromycin and moxifloxacin against bacterial isolates causing conjunctivitis. Curr Med Res Opin. 2007;23(9):2241–9. https://doi.org/10.1185/030079907X226276.
Oydanich M, Dingle TC, Hamula CL, Ghisa C, Asbell P. Retrospective report of antimicrobial susceptibility observed in bacterial pathogens isolated from ocular samples at Mount Sinai Hospital, 2010 to 2015. Antimicrob Resist Infect Control. 2017;6:29. https://doi.org/10.1186/s13756-017-0185-0.
Peng MY, Cevallos V, McLeod SD, Lietman TM, Rose-Nussbaumer J. Bacterial keratitis: isolated organisms and antibiotic resistance patterns in San Francisco. Cornea. 2018;37(1):84–7. https://doi.org/10.1097/ICO.0000000000001417.
Peterson JC, Durkee H, Miller D, et al. Molecular epidemiology and resistance profiles among healthcare- and community-associated Staphylococcus aureus keratitis isolates. Infect Drug Resist. 2019;12:831–43. https://doi.org/10.2147/IDR.S190245.
Sand D, She R, Shulman IA, Chen DS, Schur M, Hsu HY. Microbial keratitis in Los Angeles: the Doheny Eye Institute and the Los Angeles County Hospital experience. Ophthalmology. 2015;122(5):918–24. https://doi.org/10.1016/j.ophtha.2014.11.027.
Schechter BA, Sheppard JD, Sanfilippo CM, DeCory HH, Asbell PA. An evaluation of staphylococci from ocular surface infections treated empirically with topical besifloxacin: antibiotic resistance, molecular characteristics, and clinical outcomes. Ophthalmol Ther. 2020;9(1):159–73. https://doi.org/10.1007/s40123-019-00223-y.
Schimel AM, Miller D, Flynn HW Jr. Endophthalmitis isolates and antibiotic susceptibilities: a 10-year review of culture-proven cases. Am J Ophthalmol. 2013;156(1):50-52.e1. https://doi.org/10.1016/j.ajo.2013.01.027.
Slean GR, Shorstein NH, Liu L, Paschal JF, Winthrop KL, Herrinton LJ. Pathogens and antibiotic sensitivities in endophthalmitis. Clin Exp Ophthalmol. 2017;45(5):481–8. https://doi.org/10.1111/ceo.12910.
Stringham JD, Relhan N, Miller D, Flynn HW Jr. Trends in fluoroquinolone nonsusceptibility among coagulase-negative Staphylococcus isolates causing endophthalmitis, 1995–2016. JAMA Ophthalmol. 2017;135(7):814–5. https://doi.org/10.1001/jamaophthalmol.2017.1826.
Truong DT, Bui M-T, Memon P, Cavanagh HD. Microbial keratitis at an urban public hospital: a 10-year update. J Clin Exp Ophthalmol. 2015;6(6):498. https://doi.org/10.4172/2155-9570.1000498.
Yeh DL, Stinnett SS, Afshari NA. Analysis of bacterial cultures in infectious keratitis, 1997 to 2004. Am J Ophthalmol. 2006;142(6):1066–8. https://doi.org/10.1016/j.ajo.2006.06.056.
Schimel AM, Miller D, Flynn HW Jr. Evolving fluoroquinolone resistance among coagulase-negative Staphylococcus isolates causing endophthalmitis. Arch Ophthalmol. 2012;130(12):1617–8. https://doi.org/10.1001/archophthalmol.2012.2348.
Chang DF, Braga-Mele R, Henderson BA, Mamalis N, Vasavada A, ASCRS Cataract Clinical Committee. Antibiotic prophylaxis of postoperative endophthalmitis after cataract surgery: results of the 2014 ASCRS member survey. J Cataract Refract Surg. 2015;41(6):1300–5. https://doi.org/10.1016/j.jcrs.2015.01.014.
Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Atlanta: U.S. Department of Health and Human Services, CDC; 2019.
Diekema DJ, Pfaller MA, Shortridge D, Zervos M, Jones RN. Twenty-year trends in antimicrobial susceptibilities among Staphylococcus aureus from the SENTRY Antimicrobial Surveillance Program. Open Forum Infect Dis. 2019;6(suppl 1):S47–53. https://doi.org/10.1093/ofid/ofy270.
Asbell PA, Sanfilippo CM, DeCory HH. Trends in antibiotic resistance over time among staphylococci collected 2009–2019 in the ARMOR study. IOVS. 2020;61:4885.
Lalitha P, Srinivasan M, Manikandan P, et al. Relationship of in vitro susceptibility to moxifloxacin and in vivo clinical outcome in bacterial keratitis. Clin Infect Dis. 2012;54(10):1381–7. https://doi.org/10.1093/cid/cis189.
Kaye S, Tuft S, Neal T, et al. Bacterial susceptibility to topical antimicrobials and clinical outcome in bacterial keratitis. Invest Ophthalmol Vis Sci. 2010;51(1):362–8. https://doi.org/10.1167/iovs.09-3933.
Speaker MG, Milch FA, Shah MK, Eisner W, Kreiswirth BN. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology. 1991;98(5):639–49. https://doi.org/10.1016/s0161-6420(91)32239-5.
Holland EJ, McDonald MB, Parekh JG, Sheppard JD. Antibiotic resistance in acute postoperative endophthalmitis. Ophthalmology. 2014;121(11 suppl):S1–9. https://doi.org/10.1016/j.ophtha.2014.06.049.
Cavuoto K, Zutchi D, Karp CL, Miller D, Feuer W. Update on bacterial conjunctivitis in South Florida. Ophthalmology. 2008;115(1):51–6. https://doi.org/10.1016/j.ophtha.2007.03.076.
Chiquet C, Maurin M, Altayrac J, et al. Correlation between clinical data and antibiotic resistance in coagulase-negative Staphylococcus species isolated from 68 patients with acute post-cataract endophthalmitis. Clin Microbiol Infect. 2015;21(6):592e1-593.e1-e8. https://doi.org/10.1016/j.cmi.2015.01.028.
Olson R, Donnenfeld E, Bucci FA, et al. Methicillin resistance of Staphylococcus species among health care and nonhealth care workers undergoing cataract surgery. Clin Ophthalmol. 2010;4:1505–14. https://doi.org/10.2147/OPTH.S14333.
Fintelmann RE, Hoskins EN, Lietman TM, et al. Topical fluoroquinolone use as a risk factor for in vitro fluoroquinolone resistance in ocular cultures. Arch Ophthalmol. 2011;129(4):399–402. https://doi.org/10.1001/archophthalmol.2011.45.
Acknowledgements
In Memorium
The authors are saddened by the passing of their esteemed colleague, Dr. Roger Wilmer Beuerman, whom they would like to acknowledge for his valuable contributions during the early stages of manuscript development.
Funding
The journal’s Rapid Service Fees, Medical Writing, and Editorial Assistance was funded by Bausch + Lomb, a division of Bausch Health US, LLC.
Medical Writing, Editorial, and Other Assistance
The authors thank the following individuals who provided support for this paper: Kay Meyers of Bausch Health US, LLC, for performing the literature searches, and Sandra Westra, PharmD through Churchill Communications (Maplewood, NJ), for medical writing assistance.
Prior Presentation
Portions of this paper were presented at the Women in Ophthalmology 2021 Summer Symposium as a poster presentation with interim findings.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All named authors provided critical interpretation of the data for presentation in this review and reviewed, edited, and approved all versions of the manuscript. All named authors are affiliated with institutions which have contributed isolates for the ARMOR study.
Disclosures
Penny A. Asbell received lecture fees from Bausch Health US, LLC, in association with the 10-year ARMOR study publication. Paulo J. M. Bispo and Daniel F. Sahm have no conflicts to disclose.
Compliance with Ethics Guidelines
This review article is based on previously published studies and does not contain any new data from studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no original datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bispo, P.J.M., Sahm, D.F. & Asbell, P.A. A Systematic Review of Multi-decade Antibiotic Resistance Data for Ocular Bacterial Pathogens in the United States. Ophthalmol Ther 11, 503–520 (2022). https://doi.org/10.1007/s40123-021-00449-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-021-00449-9