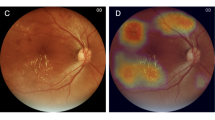
Abstract
In the presence of the ever-increasing incidence of diabetes mellitus (DM), the prevalence of diabetic eye disease (DED) is also growing. Despite many improvements in diabetic care, DM remains a leading cause of visual impairment in working-age patients. So far, prevention has been the best way to protect vision. The sooner we diagnose DED, the more effective the treatment is. Thus, diabetic retinopathy (DR) screening, especially with imaging techniques, is a method of choice for vision protection. To alleviate the burden of diabetic patients who need ophthalmic care, telemedicine and in-home testing are used, supported by artificial intelligence (AI) algorithms. This is why we decided to evaluate current image teleophthalmology methods used for DR screening. We searched the PubMed platform for papers published over the last 5 years (2015–2020) using the following key words: telemedicine in diabetic retinopathy screening, diabetic retinopathy screening, automated diabetic retinopathy screening, artificial intelligence in diabetic retinopathy screening, smartphone diabetic retinopathy testing. We have included 118 original articles meeting the above criteria, discussing imaging diabetic retinopathy screening methods. We have found that fundus cameras, stable or mobile, are most commonly used for retinal photography, with portable fundus cameras also relatively common. Other possibilities involve the use of ultra-wide-field (UWF) imaging and even optical coherence tomography (OCT) devices for DR screening. Also, the role of smartphones is increasingly recognized in the field. Retinal fundus images are assessed by humans instantly or remotely, while AI algorithms seem to be useful tools facilitating retinal image assessment. The common use of smartphones and availability of relatively cheap, easy-to-use adapters for retinal photographs augmented by AI algorithms make it possible for eye fundus photographs to be taken by non-specialists and in non-medical setting. This opens the way for in-home testing conducted on a much larger scale in the future. In conclusion, based on current DR screening techniques, we can suggest that the future practice of eye care specialists will be widely supported by AI algorithms, and this way will be more effective.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
The future role of the ophthalmologist in diabetic retinopathy (DR) care will be focused on consultations of difficult and complicated cases and their treatment. |
Telemedicine augmented by artificial intelligence (AI) will make the DR screening system more effective and cheaper, with better coverage of the diabetic population. |
The screening of DR will be done by eye technicians, general practitioners or by patients themselves supported by AI. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14589417
Introduction
Diabetes is a prevalent global disease. According to estimates, the number of diabetic people worldwide was a staggering 415 million in 2015, and this is still expected to rise and very likely to reach 642 million by 2040 [1]. Diabetic retinopathy (DR) is one of the leading causes of vision loss in working-age patients [2]. The disease usually remains asymptomatic until visual acuity decreases, but in most cases, it can be detected with retinal imaging techniques even in its early stages. It is known that the best treatment options and prognosis are for patients who present with early stages of diabetic retinopathy. Therefore, a natural solution to the problem of diabetic eye diseases seems to be diabetic retinopathy screening. The rules of screening in medicine were established in 1968 by Wilson and Jungner and were accepted by the World Health Organization (WHO) [3, 4]. The basic principles for disease screening include the following: the condition sought should be an important health problem; there should be an accepted treatment for patients with recognized disease; facilities for diagnosis and treatment should be available; there should be a recognizable latent or early symptomatic stage; there should be a suitable test or examination; the test should be acceptable to the population; the natural history of the condition, including development from latent to declared disease, should be adequately understood; there should be an agreed policy on whom to treat as patients; the cost of case finding (including diagnosis and treatment of patients diagnosed) should be economically balanced in relation to possible expenditure on medical care as a whole; and case finding should be a continuing process and not a “once and for all” project. Despite huge improvement in diabetic retinopathy detection due to screening, there is still a problem with efficiency. A useful tool for diabetic retinopathy screening is artificial intelligence (AI) used together with telemedicine techniques.
The term “telemedicine” was defined in the 1970s by Strehle and Shabde and meant “healing at a distance” [5]. WHO introduced a standardized definition of telemedicine as “the delivery of healthcare services, where distance is a critical factor, by all healthcare professionals using information and communication technologies for the exchange of valid information for diagnosis, treatment and prevention of disease and injuries, research and evaluation, and for the continuing education of healthcare providers, all in the interests of advancing the health of individuals and their communities” [6]. Telemedicine relies on information and communication technology (ICT), defined as a “diverse set of technological tools and resources used to transmit, store, create, share or exchange information. These technological tools and resources include computers, the Internet (websites, blogs, and emails), live broadcasting technologies (radio, television, and webcasting), recorded broadcasting technologies (podcasting, audio and video players and storage devices), and telephony (fixed or mobile, satellite, visio/video-conferencing, etc.)” [7]. An innovative combination of screening by means of fundus cameras, OCT and other devices with telemedicine ushered in the era of teleophthalmology, which could be applied both in ophthalmology offices and in non-eye care settings, including primary care offices. This comes with the possibility of remote grading and appropriate follow-up eye care. Growing global enthusiasm for the use of telemedicine in screening of diabetic retinopathy has led to the appearance of many publications over the last few years.
Of all diabetic patients worldwide, 75% live in low- or middle-income countries [1]. For patients living in rural environments, eye telescreening may sometimes be the only way to gain access to professional examination and treatment. Several studies found that this method produced the same clinical results as direct ophthalmological examination [8]. In public healthcare systems, the waiting time for a professional ophthalmologist appointment can be very long, owing to shortage of specialists and partially due to lack of cooperation between a patient and a doctor. Of all diabetic patients, only 34.8% have ever received a dilated fundus examination by an ophthalmologist [9]. In England, diabetic retinal screening with two-field digital mydriatic photographic examination for all diabetic patients successfully reduced the prevalence of blindness in working-age patients [10]. An additional advantage of telemedicine is its safety of use during the SARS-CoV-2 pandemic, for both patients and medical professionals. We have previously reviewed current methods and programs used in diabetic retinopathy screening adopted in different parts of the world [11]. One of the most efficient national screening programs is in the United Kingdom, where color fundus two- or one-field images are graded in specially dedicated grading centers. The first diabetic retinopathy screening program ever was started in Singapore in 1991 and was initially based on single-field Polaroid fundus photographs, and later on digital single-field retinal images [11].
The aim of this paper is to evaluate currently available imaging teleophthalmology schemes for the detection of diabetic retinopathy and to discuss the existing screening possibilities as well as the role of artificial intelligence as a diagnostic tool. The paper also aims to define the advantages and disadvantages of this examination method.
Methods
We searched the PubMed database for papers published over the last 5 years (2015–2020) using the following key words: telemedicine in diabetic retinopathy screening, diabetic retinopathy screening, automated diabetic retinopathy screening, artificial intelligence in diabetic retinopathy screening, smartphone diabetic retinopathy testing.
We selected 118 original English-language articles on the use of imaging methods in DR screening which met the above criteria for inclusion in this paper.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Techniques
Stable, Classic Non-mydriatic Fundus Cameras
Digital fundus cameras, usually non-mydriatic ones, have become the classic screening diagnostic systems. These systems of non-mydriatic fundus cameras can be stably mounted in one location and were also the most popular in our review. This is probably because they are relatively simple to operate, can be maintained by trained technicians, and so can be successfully used in the primary care setting. Non-mydriatic, non-stereo cameras were also among the first to be used in diabetic retinopathy screening, and their use is well established in the field [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. More accurate but at the same time more complicated methods have involved the use of seven-field ETDRS standard stereoscopic photographs of the eye fundus. Those images had to be taken with a wide pupil, by very experienced photographers; grading was also technically more difficult [61,62,63]. Six pairs of stereo images of each eye were taken by Park et al. [64], three pairs of stereo images were taken by Silva et al. [65], and two pairs of two-field stereo images were taken by Bursell et al. [66] and by Mansberger et al. [67].
Mobile Classic Non-mydriatic Fundus Cameras (moved from location to location)
A more effective solution, generating lower costs and allowing for wider coverage (up to 70% of the diabetic population), is the classic non-mydriatic fundus camera which can be moved from one location to another place of examination. This usually follows a previously planned scheme [68].
Mobile, On-Vehicle Hard-Mounted Diagnostic Sets
In order to optimize the use of current diagnostic resources, diagnostic sets can be hard-mounted on vehicles. Ultra-wide-field imaging is more accurate than classic non-mydriatic cameras and, when hard-mounted on a vehicle, is an even more perfect screening set [69]. Mobile screening sets have even been specially named “virtual clinics” and are based on ultra-wide-field and classic non-mydriatic cameras [50, 70]. Mobile DR screening vans have also been used in China to take care of diabetic patients [27].
Ultra-Wide-Field (UWF) Diagnostic Sets
A more effective and more accurate method of diabetic retinopathy screening is assessment by means of ultra-wide-field fundus cameras, in some cases combined with macular OCT, to improve the detection of macular edema [41,42,43,44, 41,42,43,44, 36,37,38,39, 67, 69, 71, 72]. UWF as a stereo pair was reported by Silva et al. [65].
Optical Coherence Tomography (OCT)-Based Diabetic Retinopathy Screening
Some authors recommend the use of OCT devices for more accurate and reliable detection of macular edema [23, 44, 58, 72,73,74,75,76].
Portable Fundus Cameras
The use of a portable fundus camera allows patients from rural parts of the country to be tested for the presence of diabetic retinopathy [77]. This method involves the use of a portable, non-mydriatic, handheld, lightweight digital fundus camera with a 45° field of view. It is supported by a trained nurse or technician using an educational pamphlet translated into local languages, highlighting the importance of regular eye screening. These images can be used in initial screening for the presence of disease, and can help determine whether patients require a dilated exam by an ophthalmologist [32, 78,79,80,81]. Web-based portable fundus cameras were presented by Keshvardoost et al. [82]. Zhang et al. [83] concluded in their study that handheld fundus cameras were sufficiently efficient in DR screening.
Smartphone-Based Retinal Imaging
Smartphones are nowadays the most common portable devices used worldwide. In ophthalmology they can be used for ophthalmological examination. There are several types of smartphone adapters, making it possible to see and take a picture of the eye fundus, with or without pupil dilation; these include Peek Retina [84] and D-Eye adapter, adapters for smartphones such as the iExaminer, or all-in-one devices such as the Horus Scope and Smartscope PRO [85], a wide-field smartphone fundus video camera, and CellScope Retina, a retinal imaging system [87, 88]. Smartphones are also used for monocular indirect ophthalmoscopy with application of the 20D Volk lens, and a plastic adapter to hold the lens in one piece with the phone (EyeArt) [89]. Various healthcare workers can potentially operate a smartphone-based retinal imaging device [84, 89, 90], as their use is not restricted to qualified staff [84, 86,87,88, 90]. The effectiveness, defined as the number of changes in the posterior segment identified by ophthalmological technicians as compared to ophthalmologists, was found to differ in 42.4% [90]. However, in terms of examination of the anterior part of the eye and qualification for cataract surgery, as well as the diagnosis of absence of any changes in the eye, the effectiveness is more comparable between technicians and ophthalmologists (k = 0.732, 95% CI 0.65–0.81; k = 0.642, 95% CI 0.53–0.75) [90]. The patients themselves or their families can also perform retinal imaging with a smartphone camera as in-home testing [86, 87]. The only condition is the need to buy a special adapter for a smartphone that is lightweight and easy to use; no other changes in the telephone are necessary. Good-quality pictures can be easily shared via telemedicine and analyzed remotely by an ophthalmologist or other trained grader [84, 86,87,88]. As a diagnostic and monitoring tool, smartphone detection can be particularly important in rural areas and in developing countries, where access to healthcare and qualified staff is limited and medical equipment is lacking [90, 91]. Children can also be easily examined with a smartphone device, which is lightweight and can be easily moved in front of a person’s eye [84, 88, 92, 93]. The quality of smartphone imaging is lower than that of conventional fundus imaging. More artifacts appeared in smartphone imaging when the mid- and far periphery of the retina was included in pseudophakic eyes [94]. The quality of images depends on the type of smartphone adapter, but not on the experience of the investigator [91]. It is possible to take a picture of the fundus without dilation of the pupil, but it is more time-consuming to obtain a clear image [94]. A picture of the optic disc and the macula can be taken without special adapters, after pupil dilation, using the 20D Volk lens in front of the patient’s eye [95]. A video can be taken and then a screenshot from it or a direct picture during examination [95]. Smartphone imaging is easier to perform by an unqualified examiner compared to direct ophthalmoscopy (82.3% vs. 48.5%, P < 0.0001) [96]. Another device is a special piggyback-design Fundus on Phone retina camera, which is fitted to commercially available smartphones. It is a low-weight camera (900 g), so it can be easily carried from place to place. Diabetic retinopathy grading is difficult when based on pictures taken with this camera because of their quality, but it is a useful instrument to detect changes in the fundus. The available piggyback camera has a 45° field of view, 33 mm working distance, and +20 to –20 D adjustment; it has optical magnification of ×12, and its battery lifetime is up to 7 h. A special application allows patients to store data in a folder. Pictures can be sent for evaluation using the WhatsApp application, which is a free and encrypted application [7].
Smartphone-based semiautomatic DR detection was also proposed by Saeed et al. [97], using the d-Eye sensor mounted on a mobile phone to give patients the possibility of in-home testing. The obtained images are sent via the web to an algorithm which detects hard exudates. Another example of smartphone use in DR telescreening was proposed by Toy et al. [63], who used a special adapter mounted on a mobile phone to obtain retina pictures, which could be assessed remotely.
Smartphone testing is usually easy and can be performed by non-specialists and in a non-professional setting, so it can take the form of in-home testing.
Methods of retinal image acquisition are presented in Table 1.
The Process of Photograph-Taking
Several DR telescreening models have been implemented. Retinal images are generally acquired by trained primary care physicians, technicians, ophthalmic photographers and nurses [7, 10, 12, 13, 15, 17, 18, 20, 22, 23, 25,26,27, 27,28,29,30,31, 53,54,55,56,57,58,59,60, 63,64,65, 68,69,70,71,72, 51,52,53, 53, 97,98,99,100,101, 57, 58, 58, 59, 26,27,28,29,30, 64, 64, 65, 65, 48,49,50,51,52,53,54,55, 58,59,60, 63,64,65,66,67, 81, 82, 82, 83, 79,80,81,82,83].
Grading Methods
Human Grading
The data collected in medical centers from diabetic patients during camera-based fundus examinations are sent for diabetic retinopathy detection to experts including ophthalmologists or retinal specialists [13, 16, 18, 20, 21, 23, 24, 26,27,28,29, 32, 33, 35, 42,43,44,45, 47,48,49,50,51,52, 54, 56, 64, 67,68,69,70,71,72,73,74,75, 79, 81,82,83], certified technicians and nurses; in more complicated cases, the data are reevaluated by retinal specialists [12, 15, 20, 31, 41, 44, 59, 63, 66, 78, 102].
To reduce problems with under- or over-evaluation, two or more readers can participate in examinations [53, 80]. There can also be two or more masked reviewers, who check the same data, and a third reviewer or more for inconsistent decisions [18, 52, 80]. Prior to commencement of work, some readers have completed a special training program or obtained a certificate for retinal evaluation [17, 18, 48, 52].
Artificial Intelligence
AI is a technology created to mimic the perception and information processing of the human brain by a machine to make objective decisions [73]. It can improve the quality and efficacy of ophthalmic examinations as well as reduce the costs. Over the past 5 years, artificial intelligence systems have undergone dynamic changes and are expected to be evaluated in the future. AI can be divided into three types: supervised, semi-supervised and unsupervised [100]. The taxonomy is based on the possibility of adjustment of some parameters during the training phase, which are collected in response to a specific performance, until acceptable compliance is achieved. AI can use multiple retinal detectors to find special features on the retina which were pathognomonic for diabetic retinopathy, for example hemorrhages, exudates, microaneurysms, and nonlinear remodeling of the outer retinal layers. The detected changes are then classified by the system as normal or abnormal and the final output is generated. This type of AI is categorized as lesion-based, because it is used for detecting relevant abnormal lesions. Artificial intelligence can also integrate one or more multilayer neural networks which are trained to associate diagnostic outputs on disease level, for instance for a retinal image. Each image is analyzed and compared to a large corresponding output in a training set. Based on this pixel data of changes characteristic of diabetic retinopathy, the system learns how to grade images. In most studies, AI is based on color fundus examination imaging [73]. Optical coherence tomography (OCT) and OCT angiography (OCTA) have also been integrated into AI systems, but it was very challenging to create a database for a large, multicenter system [73].
In order to classify images using artificial intelligence, various quantitative parameters are needed. In one study, OCTA vessel maps and skeletal maps were extracted from OCT scans and vascular features were measured, including blood vessel tortuosity (BVT), blood vascular caliber (BVC), vessel perimeter index (VPI), blood vessel density (BVD), foveal avascular zone (FAZ) area (FAZ-A) and FAZ contour irregularity (FAZ-CI) [73]. All these parameters were recalculated and compared between healthy patients and patients diagnosed with diabetic retinopathy and sickle cell retinopathy [82]. The optimal feature combination directly correlated with the most significant morphological changes in the retina. It also had limitations, however, including severe artifacts, segmentation errors and errors in reconstruction, which had to be identified and eliminated.
The IDX-DR was developed from the Iowa Detection Program (IDP) and is based on convolutional neural networks [103,104,105]. Through the addition of new deep learning features to IDP, specificity improved from 54.9% for IDP to 87% for IDX-DR, while the high sensitivity of IDP and IDX-DR remained unchanged [103]. This AI algorithm also achieved Food and Drug Administration (FDA) approval as the first FDA-approved fully autonomous AI diagnostic system [106]. This algorithm is designed to work with the Topcon non-mydriatic NW400 fundus camera to take macula- and disc-centered pictures of each eye.
Retmarker DR is a Portuguese machine learning algorithm for DR detection as “disease” or “no disease,” and requires subsequent human verification [107]. This system is able to compare current retina pictures with previously taken pictures and to assess worsening or improvement of DR state [108]. Its sensitivity for referable DR is 85%. This algorithm is a CE-marked Class IIa medical device.
The EyeArt system, a CE-marked IIa medical device developed by Eyenuk, Inc., Los Angeles, CA, USA, is cloud-based with telemedicine software. The algorithm automatically excludes pictures of inadequate quality and offers the possibility for macroaneurysm turnover assessment. Its screening sensitivity is 91.7% (95% CI 91.3–92.1%) and specificity is 91.5% (95% CI 91.2–91.7%) [5]. Eyenuk, Inc. also offers another algorithm, EyeMark, for macroaneurysm turnover assessment. This software can also work on smartphone app-based images (tested on a Remidio Fundus on Phone device), with 95.8% sensitivity for any DR and specificity of 80.2% [6].
Google Inc. developed a convolutional neural network-based algorithm for automatic DR detection [7]. This system can be tweaked for higher specificity (93.9%) and sensitivity (96.1%) for referable DR prediction.
Singapore SERI-NUS, presented by Ting et al. [109], is a deep learning-based algorithm for DR detection, with sensitivity of 90.5% and specificity of 91.6% for referable DR. This software was also used for the detection of suspected glaucoma and age-related macular degeneration (AMD).
The Bosch DR algorithm is a convolutional neural network-based AI system used on the Bosch Mobile Eye Care fundus cameras with the following output: disease/no disease and picture quality assessment. This system showed sensitivity of 91% and specificity of 96% [61].
RetinaLyze, a CE-marked Class I device software system with end-to-end encryption, is an algorithm for automatic eye fundus image analysis for DR, glaucoma and AMD detection with website-based assessment. Its sensitivity for DR detection is 93.1% and specificity is 71.6% [110,111,112].
Gargeya and Leng presented their DR detection deep learning-based algorithm [113]. They used this system both on desktop hardware and on an iPhone 5 and achieved sensitivity of 93% and specificity of 87% for disease and no disease diagnosis.
Li et al. tested their deep learning-based algorithm for DR detection. They presented external validation tests for referable DR with 92.5% sensitivity and 98.5% specificity [114].
Alam et al. [73] proposed a supervised machine learning-based approach in which a support vector machine (SVM) classifier was trained to evaluate diabetic retinopathy with multi-task AI classification, using quantitative OCTA features. There were three steps to achieve validation of the OCTA image. The first was image data acquisition. The second step was the hierarchical backward elimination technique supported by SVM, which was used to identify an optimal feature combination for the best diagnostic accuracy and the most efficient classification. As the third step, multilayer hierarchical tasks were performed to create classification of normal retina and disease, inner disease classification for diabetic retinopathy and sickle cell retinopathy, and grading of each disease.
Stevenson et al. [115] created a convolutional neural network-based algorithm for color fundus photo-based detection of DR, glaucoma and AMD. The average sensitivity for each disease was 75% and average specificity was 89%.
Kanagasingam et al. [22] checked the usefulness of AI in diabetic eye fundus photograph grading in Australia. They created their own AI system for (1) the detection of DR versus no DR, (2) the detection of hard exudates and microaneurysms, and (3) assessment of the severity of DR, based on the International Clinical Diabetic Retinopathy Disease Severity Scale criteria. They achieved 92% specificity and a positive predictive value of 12%.
Saha et al. [24] proposed their own AI (deep convolution neural network) system to instantly check the quality of eye retina pictures meant for telescreening of DR, which saved time waiting for the final diagnosis by eliminating the need for repeated examinations. The results were obtained as “accept” or “reject” an image. The “reject” result meant a retake of the fundus picture. The authors achieved 97% agreement between the AI algorithm and control human grading (accuracy of 100%).
Pedrosa et al. [57] presented a multidisciplinary collaborative platform based on machine learning to create an algorithm to help diagnose DR by evaluating image quality, discarding healthy eyes and DR grading. The results were presented as average time needed for image evaluation, with shorter time for bad image quality and longer time for good quality. The current results can be accessed at http://demo.dicoogle.com/screen-dr.
Walton et al. [60] tested the efficacy of an automated algorithm based on a neural network (Intelligent Retinal Imaging System, IRIS) for DR detection, reported as “referral” or “observation.” The authors concluded that their software had high sensitivity (66% compared to human grading), with specificity of 72.8% and a very low rate of false negatives (2%), so the diagnosis could be an effective alternative to human grading.
Remote Grading
The obtained retinal image data were assessed remotely. It was possible to transfer the data via web and receive immediate online diagnosis [25, 29]. There was also a special teleretinal screening software platform used [16, 22, 26, 27, 29, 30, 35, 37, 44, 47, 51, 54, 56, 59, 65,66,67, 69, 70, 72, 75, 81, 97] or, alternatively, access to all information could be obtained through a special account on a medical platform.
The length of time the patients had to wait for the result of the examination was also important. It could be obtained almost in real time [77], or it could be minutes [25, 29], hours or days [38]. If direct contact was not possible through a teleretinal platform, the patients had to wait for their examination results, and they were informed whether an additional appointment in an ophthalmology center was needed. This could be done by phone, letter or email sent to the patient and to their primary care physicians [38] or just to their primary care doctor; the results were then shown to the patient during the next follow-up appointment.
Limitations and Positive Aspects of Telescreening of DR
There were many limitations related to telemedicine highlighted by numerous researchers.
The authors of several publications reported some problems in obtaining retinal images of good quality. Reasons for that included a small pupil of the examined eye and the need to take images after mydriasis [25, 82]. This was why some authors suggested screening with a wide pupil [14, 41]. Another cause of low-quality photographs was poor transparency of optic media caused by cataract and other opacities [13, 14, 21, 23, 25, 80, 82, 97,98,99,100].
There were also significant problems regarding telemedicine programs associated with a growing demand for trained/certified eye fundus photographers [81] and image readers [18, 63]. As a solution to that problem, training in picture-taking could be offered to nurses and technicians [12, 15, 18, 22, 23, 27, 31,32,33,34, 38, 42,43,44,45, 47, 48, 50, 52,53,54,55,56,57, 60, 63, 64, 68, 70,71,72, 74, 79,80,81, 98,99,100]. One promising solution was in-home testing, where patients themselves took pictures of their retinas [97]. Grading could then be performed by certified technicians and nurses [71, 101, 102] or by AI, replacing retina specialists [14, 43, 60, 73, 100].
The need for advanced and expensive screening devices, such as fundus cameras and special software, was quite a serious problem [14, 17, 46, 65, 73, 78, 82, 99]. Portable fundus cameras [32, 48, 82] and complete mobile sets could lower the total cost of telescreening by optimizing currently available resources [28, 52, 69, 70, 72]. However, some studies noted limited sensitivity and specificity of smartphone examinations and lack of applications dedicated to screening. AI helped in the use of low-quality portable screening devices for high-quality assessments [117]. Smartphones needed a wide pupil for good-quality images [38, 63, 73]
Some authors suggested that classic 45° single-, two- or three-field images were insufficient to detect all diabetic retinopathies [13, 14, 16, 20, 27, 28, 35, 38, 45, 47, 49, 51,52,53,54, 62, 66, 70, 73,74,75, 80,81,82, 99, 100], and ultra-wide-field imaging (200° angle of view) was needed for effective detection [41, 44, 46, 65, 66, 69, 71, 72, 74].
A serious problem related to telescreening was a low percentage of follow-up of vision-threatening diabetic retinopathy (VTDR) due to social and educational factors such as low education level, limited elderly mobility, transport difficulties, loneliness, depression, financial problems, distrust of the recommended hospital, making the referral appointment, fear of examination and treatment, no response to previous treatment and no sensation of being ill [28, 29, 56, 116,117,118]. In an attempt to reduce the negative effects of poor education, telescreening was combined with diabetes education, which improved diabetes control [24, 26, 39].
There were also problems with telescreening for DR, such as the need for expensive and complex systems to detect all stages of DR [44] or a long waiting time for the final diagnosis after image acquisition [38, 99]. Here, AI algorithms could offer a solution as an effective alternative to human grading [14, 43, 60, 73]. Automated systems are cost-effective [40] and can check the image quality instantaneously, thereby saving money and time otherwise needed for follow-up appointments [14, 24, 61]. Those systems reduced waiting time for DR screening, provided more regular screening [14, 29, 30, 43, 78] and were highly effective (100,000 patients in 45 h) [14, 43]. Some authors noted the high quality of handheld fundus cameras [32, 48, 83]. Virtual (driven) clinics (mobile set) offered the standard of eye care of a clinical setting and allowed optimization of currently used resources, and so reduced the total cost of telescreening [28, 52, 69, 70, 72]. Finally, some researchers noted the lack of an integrated virtual platform which could bring all the necessary data together [75].
Some authors found no positive influence of teleretinal diabetic retinopathy screening on patients’ access to eye examination [15], and others noted limited coverage of telemedicine and the need for co-payment for some examinations [16]. Many studies reached quite opposite conclusions and mentioned high accuracy in diabetic retinopathy diagnostics, especially in treatable patients [21, 54, 79, 82]. Many authors pointed to the significant role of screening in the prevention of unnecessary referrals (reduction by 75%) [14, 19, 30, 73, 82]. Diabetic retinopathy screening covered close to 70% of diabetic patients in rural regions [13, 14, 68, 72] and improved the quality of medical care, especially in remote areas [25, 33, 72, 78, 99]. Studies found that a higher percentage of diabetic patients received eye care with telemedicine compared to traditional surveillance [13, 14, 17, 59, 67, 79, 118, 119], along with a high satisfaction rate among screened patients [26, 47, 62] and among professionals conducting the examinations [26, 47].
Recommendations for further examinations (follow-up) were assessed [16, 24, 32, 35, 39, 47, 54, 55, 71]. Better-educated patients preferred comprehensive eye examination to fundus camera screening test only [28], and some authors concluded that telemedicine should be targeted toward patients with poor access to medical care [23, 120]. Non-ocular diseases and ocular diseases found incidentally by means of DR telescreening and AI included AMD, glaucoma, hypertensive retinopathy and disc pallor [21, 28, 34, 38, 45, 49, 50, 55, 64, 75, 81].
In 2017, Xiao et al. presented a comprehensive teleretinal eye treatment plan with SMS [short messaging service] patient information on the date of eye examination. To improve the quality of telescreening, internal self-checking of image system quality was assessed [99], and a development scheme was used to design a DR telemedicine screening program [37]. To improve access to DR screening, local women’s self-help groups were tasked with its implementation [74].
According to some authors, the diagnosis of diabetic macular edema (DME) should not be made only on the basis of color fundus photographs, but should also include OCT scans [76]. Screening performed with OCT provided diagnostic characteristics of OCT such as epiretinal membrane (ERM), glaucoma, AMD and vitelliform degeneration [34]. On the other hand, maintenance of fundus camera/OCTA was time-consuming [73, 82], AI software was not integrated with OCT devices [34, 73], and AI for OCTA/retinal images needed a huge database of 100,000–1,000,000 scans [14, 73].
The positive and negative aspects are presented in Tables 2 and 3.
Discussion
The predicted 50% rise in diabetes prevalence by 2040 [1], particularly in developing countries, will lead to an increased demand for ophthalmological testing in diabetic patients. This in turn will put a greater burden on the system of ophthalmic care that relies mainly on direct examination, and will significantly challenge its efficiency. Screening of diabetic retinopathy is commonly known to be the best method for preventing serious complications of diabetes. If screening is to cover the whole population, an efficient screening system is needed, together with a well-functioning specialist care structure to treat more advanced diabetes-related ophthalmic complications. Teo et al. found the prevalence of VTDR to be 7.26%, ranging from 14.3% in Africa to almost 2.97% in Southeast Asia. The authors also calculated an average of 7.16 ophthalmologists per 1000 patients with VTDR globally, with significant differences depending on the region. In rich Europe, the average number of ophthalmologists was 18.03, while in poor regions of Africa the figure was as low as 0.91 ophthalmologists for every 1000 VTDR patients [121].
Screening may be performed by humans only (e.g. an English model [126]), it may rely on remote image analysis using AI [103,104,105, 108], or it may be based on a mixed model, using both humans and AI [107]. Screening tests may be conducted directly by an ophthalmologist (a method characterized by high accuracy, but requiring considerable time and specialists, which now and in the future offers no possibility for screening the whole diabetic population). A solution to this problem is an examination conducted with color fundus images or OCT, further assessed by ophthalmologists, i.e. using telemedicine (the method recognized by WHO), which in this case is teleophthalmology. This is when a limited number of specialists provide care for a much larger patient population. In developed countries, screening programs receive substantial funding, and consequently their efficiency is higher; they mostly use stationary diagnostic centers, offering care to a larger part of the diabetic population (the UK, Denmark, the Netherlands, Singapore, Sweden). In developing countries, however, where the costs of implementing efficient screening models for DR are relatively high, various forms of mobile diagnostic units are used to reach patients [11].
A classic definition of telemedicine proposed in the 1970s by Strehle and Shabde should now be modified to encompass the use of AI. Based on the assumption that the classic form of teleophthalmology involves a remote patient–ophthalmologist relation, the concept of telemedicine should be expanded to include an indirect contact between a patient and the doctor of another specialty, such as a general practitioner (GP), or even a trained technician, and only later, if the situation requires, an ophthalmologist. Similarly, screening supported by AI for the processing of eye fundus images (computer-aided diagnostics) which are submitted to ophthalmologists only in justified cases can also be regarded as a form of telemedicine, although mostly implemented locally, without sending patient’s data (online access for software). Telemedicine making use of advanced imaging techniques such as ultra-wide-field imaging and OCT use, supported by AI algorithms, may be used to detect not only DR but also co-occurring diseases such as AMD, glaucoma, retinal degeneration of another etiology, choroidal nevi and intraocular tumors.
The disadvantages of telemedicine include its technical limitations (it is not possible to investigate all aspects of the disease and verify them remotely); other limitations may include the initial costs of the diagnostic equipment, software, training and staff-related costs. Another problem may be the provision of screening services on a daily basis, such as identifying patients and making their first and follow-up appointments, and patient post-screening compliance.
A good solution for reducing the high costs of DR screening is customizing intervals between routine check-up appointments. According to the standard recommendation, for a diabetic presenting no eye problem, a check-up should take place every year or every 2 years, but on evaluation of DR risk factors such as blood glucose levels, blood pressure, gender, type of DM and duration of DM, the interval may be extended even to 4 years [11, 122]. The RetinaRisk.com platform has successfully implemented this solution, providing efficient service and safety for patients. It reduces screening costs by extending the length between examinations to more than 1 year for mild stages of DR. This helps to reduce the burden of unnecessary ophthalmologist appointments.
In the era of common use of smartphones and special adapters with relatively cheap software, this technique facilitates self-screening of DR (in-home testing) [86, 87, 97], at least as preliminary testing. Therefore, it would make access to specialist eye care much easier for middle- and low-income patients. In-home testing, in the time of the COVID-19 pandemic, could become a method of choice for DR screening.
Telemedicine platforms may fill many of the gaps in access to DR screening, offering a cost-effective way to detect preventable disease in a primary care setting [123,124,125].
However, improvements in retinal screening do not consistently lead to the prevention of blindness from VTDR. In some settings, as many as 80% of patients found to have VTDR through retinal screening will not complete follow-up ophthalmological evaluation and treatment recommendations [11].
Kozak et al. [98] used telemedicine in their study not only to evaluate retinal images of diabetic patients; they went a step further and planned laser therapy for patients with macular edema. In a referral center, grid patterns were planned on the pictures sent for each patient and sent back to the examination center to perform treatment. To facilitate the process of sending data between the diagnostic centers, a special web-based platform was used. This made it possible to send, store and visualize photographs. Patient history and medical pictures collected in the medical center were sent via the web to an evaluator for diagnosis and referral [13]. Personal access was required to approach the database [13].
Conclusions
The future of ophthalmic care seems to be remote eye examination done by a technician and aided by AI, with in-home self-testing as a preliminary test. Pre-specialist diagnostics will automatically identify healthy eyes, make a diagnosis and recommend a referral only if necessary. This will make the system less dependent on highly specialized and expensive human resources and will indirectly lead to optimal use of current resources.
References
Ogustova K, da Rocha FJ, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40.
Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015;2:17.
Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–36.
Wilson J, Jungner G. The principles and practice of screening for disease, Public Health Papers. Geneva: WHO; 1968.
Tufail A, Kapetanakis VV, Salas-Vega S, Egan C, Rudisill C, Owen CG, et al. An observational study to assess if automated diabetic retinopathy image assessment software can replace one or more steps of manual imaging grading and to determine their costs effectiveness. Health Technol Assess (Rockv). 2016;20:1–72 (Xxviii).
Rajalakshmi R, Subashini R, Anjana RM, Mohan V. Automated diabetic retinopathy detection in smartphone-based fundus photography using artificial intelligence. Eye. 2018;32:1138.
Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316:2402–10.
Sreelatha OK, Ramesh SV. Teleophthalmology: improving patient outcomes? Clin Ophthalmol. 2016;10:285–95.
Verdager J, Martínez F, Barría, F. Actualización de la Guía Clínica de Retinopatía Diabética. International Council of Ophthalmology 2016. http://www.icoph.org/dynamic/attachments/resources/guiaclinicaretinopatiadiabetica2016.pdf.
Zimmer-Galler IE, Kimura AE, Gupta S. Diabetic retinopathy screening and the use of telemedicine. Curr Opin Ophthalmol. 2015;26(3):167–72 (10 Ministry of Health Chile. Community health records with a focus on social determinants, Bio-Bio Region, 2015).
Pieczynski J, Grzybowski A. Review of diabetic retinopathy screening methods and programmes adopted in different parts of the world. European Ophthalmic Review. 2015;9:49–55.
Malerbi FK, Carneiro ABM, Katz M, et al. Retinal exams requested at primary care unit: indications, results and alternative strategies of evaluation. Einstein (Sao Paulo). 2019;18:913. https://doi.org/10.31744/einstein_journal/2020GS4913 (eCollection 2020).
Avendaño-Veloso A, Parada-Hernández F, González-Ramos R, Dougnac-Osses C, Carrasco-Sáez C, Scanlon PH. Teleophthalmology: a strategy for timely diagnosis of sight-threatening diabetic retinopathy in primary care, Concepción. Chile Int J Ophthalmol. 2019;12(9):1474–8.
Bhaskaranand M, Ramachandra C, Bhat S, Cuadros J, Nittala MG, Sadda SR, Solanki K. The value of automated diabetic retinopathy screening with the EyeArt system: a study of more than 100,000 consecutive encounters from people with diabetes. Diabetes Technol Ther. 2019;21(11):635–43. https://doi.org/10.1089/dia.2019.0164 (Epub 2019 Aug 7).
Mamillapalli CK, Prentice JR, Garg AK, Hampsey SL, Bhandari R. Implementation and challenges unique to teleretinal diabetic retinal screening (TDRS) in a private practice setting in the United States. J Clin Transl Endocrinol. 2019;19:100214. https://doi.org/10.1016/j.jcte.2019.100214.
Ramchandran RS, Yilmaz S, Greaux E, et al. Patient perceived value of teleophthalmology in an urban, low income us population with diabetes. PLoS ONE. 2020;15(1):e0225300. https://doi.org/10.1371/journal.pone.0225300 (eCollection 2020).
Joseph S, Kim R, Ravindran RD, Fletcher AE, Ravilla TD. Effectiveness of teleretinal imaging-based hospital referral compared with universal referral in identifying diabetic retinopathy: a cluster randomized clinical trial. JAMA Ophthalmol. 2019;137(7):786–92.
Liu Y, Rajamanickam VP, Parikh RS, Loomis SJ, Kloek CE, Kim LA, Hitchmoth DL, Song BJ, Xerras DC, Pasquale LR. Diabetic retinopathy assessment variability among eye care providers in an urban teleophthalmology program. Telemed J E Health. 2019;25(4):301–8. https://doi.org/10.1089/tmj.2018.0019 (Epub 2018 Jul 24).
Modjtahedi BS, Theophanous C, Chiu S, Luong TQ, Nguyen N, Fong DS. Two-year incidence of retinal intervention in patients with minimal or no diabetic retinopathy on telemedicine screening. JAMA Ophthalmol. 2019;137(4):445–8 (Published online 2019 Feb 7).
Cunha LP, Figueiredo EA, Araújo HP, et al. Mydriatic fundus retinography in screening for diabetic retinopathy: agreement between family physicians, general ophthalmologists, and a retinal specialist. Front Endocrinol (Lausanne). 2018;9:251. https://doi.org/10.3389/fendo.2018.00251.
Safi S, Ahmadieh H, Katibeh M, Yaseri M, Nikkhah H, Karimi S, Nourinia R, Tivay A, Zareinejad M, Azarmina M, Ramezani A, Moradian S, et al. Modeling a telemedicine screening program for diabetic retinopathy in Iran and implementing a pilot project in Tehran Suburb. Res Article Open Access. 2019. https://doi.org/10.1155/2019/2073679.
Kanagasingam Y, Xiao D, Vignarajan J, et al. Evaluation of artificial intelligence-based grading of diabetic retinopathy in primary care. JAMA Netw Open. 2018;1(5):e182665. https://doi.org/10.1001/jamanetworkopen.2018.2665.
Yaslam M, Al Adel F, Al-Rubeaan K, AlSalem RK, Alageel MA, Alsalhi A, AlNageeb D, Youssef AM. Non-mydriatic fundus camera screening with diagnosis by telemedicine for diabetic retinopathy patients with type 1 and type 2 diabetes: a hospital-based cross-sectional study. Ann Saudi Med. 2019;39(5):328–36. https://doi.org/10.5144/0256-4947.2019.328 (Epub 2019 Oct 3).
Saha SK, Fernando B, Cuadros J, et al. Automated Quality assessment of colour fundus images for diabetic retinopathy screening in telemedicine. J Digit Imaging. 2018;31(6):869–78. https://doi.org/10.1007/s10278-018-0084-9 (PMID: 29704086).
Crossland L, Askew D, Ware R, Cranstoun P, Mitchell P, Bryett A, Jackson C. Diabetic retinopathy screening and monitoring of early stage disease in Australian general practice: tackling preventable blindness within a chronic care model. J Diabetes Res. 2016;16:8405395. https://doi.org/10.1155/2016/8405395 (Published online 2015).
Serrano CI, Shah V, Abràmoff MD, et al. Use of expectation disconfirmation theory to test patient satisfaction with asynchronous telemedicine for diabetic retinopathy detection. Int J Telemed Appl. 2018. https://doi.org/10.1155/2018/7015272 (eCollection 2018).
Wong IYH, Ni MY, Wong IOL, et al. Saving sight in China and beyond: the lifeline express model. BMJ Glob Health. 2018;3(4):e000766. https://doi.org/10.1136/bmjgh-2018-000766 (eCollection 2018).
Dóra JE, Dóra JS, Greg R, Kirby P, Edit P, László N, Facskó A, Morten CM, Petrovski BE. Diabetic retinopathy screening using telemedicine tools: pilot study in Hungary. Res Art Open Access. 2016;4529824:9. https://doi.org/10.1155/2016/4529824.
Sheba MG, Erin Moran H, Fish A, Daskivich LP, Ogunyemi OI. Understanding the Knowledge Gap Experienced by U.S. Safety Net Patients in Teleretinal Screening. In: AMIA Annu Symp Proc. 2017;2016:590–9.
Daskivich LP, Vasquez C, Martinez C Jr, et al. Implementation and evaluation of a large-scale teleretinal diabetic retinopathy screening program in the Los Angeles County Department of Health Services. JAMA Intern Med. 2017;177(5):642–9. https://doi.org/10.1001/jamainternmed.2017.0204.
Litvin TV, Weissenberg CR, Daskivich LP, Zhou Q, Bresnick GH, Cuadros JA. Improving accuracy of grading and referral of diabetic macular edema using location and extent of hard exudates in retinal photography. J Diabetes Sci Technol. 2016;10(2):262–70.
Davila JR, Sengupta SS, Niziol LM, et al. Predictors of photographic quality with a handheld nonmydriatic fundus camera used for screening of vision-threatening diabetic retinopathy. Ophthalmologica. 2017;238(1–2):89–99. https://doi.org/10.1159/000475773 (Epub 2017 Jul 5).
Hatef E, Alexander M, Vanderver BG, et al. Assessment of annual diabetic eye examination using telemedicine technology among underserved patients in primary care setting. Middle East Afr J Ophthalmol. 2017;24(4):207–12. https://doi.org/10.4103/meajo.MEAJO_19_16.
Invernizzi A, Bevilacqua MT, Cozzi M, Bianchi C, Pagani A, Cigada M, Staurenghi G. Diabetic retinopathy screening: the first telemedical approach in an Italian Hospital. Observational Study. Eur J Ophthalmol. 2016;26(4):369–74. https://doi.org/10.5301/ejo.5000719 (Epub 2015 Dec 14).
Jani PD, Forbes L, Choudhury A, et al. Evaluation of diabetic retinal screening and factors for ophthalmology referral in a telemedicine network. JAMA Ophthalmol. 2017;135(7):706–14. https://doi.org/10.1001/jamaophthalmol.2017.1150.
Maa AY, Patel S, Chasan JE, Delaune W, Lynch MGG. Retrospective evaluation of a teleretinal screening program in detecting multiple nondiabetic eye diseases. Telemed e-Health. 2017. https://doi.org/10.1089/tmj.2016.0039 (Original Research. Published Online:1 Jan 2017).
Safdari R, Langarizadeh M, Ramezani A, et al. Development of a store-and-forward telescreening system of diabetic retinopathy: lessons learned from Iran. J Diabetes Metab Disord. 2018;17(1):31–6. https://doi.org/10.1007/s40200-018-0335-9 (eCollection 2018 Jun).
Murchison AP, Friedman DS, Gower EW, Haller JA, Lam BL, Lee DJ, McGwin G Jr, Owsley C, Saaddine J. Insight study group a multi-center diabetes eye screening study in community settings: study design and methodology. Ophthalmic Epidemiol. 2016;23(2):109–15. https://doi.org/10.3109/09286586.2015.1099682 (Epub 2016 Mar 7).
Aiello LP, Cavallerano J, Sun J, et al. Long-term effect on HbA1c in poorly controlled diabetic patients following nonmydriatic retinal image review at the time of endocrinology visit. Telemed J E Health. 2020. https://doi.org/10.1089/tmj.2019.0239.
Rico-Sergado L, Pérez-Canales JL, Pérez-Santonja JJ. Effect of visual impairment on teleretinal imaging for diabetic retinopathy screening. Ophthalmic Surg Lasers Imaging Retina. 2016;47(1):42–8. https://doi.org/10.3928/23258160-20151214-06.
Boucher MC, Qian J, Brent MH, et al. Steering Committee for Tele-Ophthalmology Screening, Canadian Retina Research Network. Evidence-based Canadian guidelines for tele-retina screening for diabetic retinopathy: recommendations from the Canadian Retina Research Network (CR2N) Tele-Retina Steering Committee. Can J Ophthalmol. 2020;55(1S1):14–24. https://doi.org/10.1016/j.jcjo.2020.01.001.
Seoud L, Hurtut T, Chelbi J, Cheriet F, Langlois JMP. Red Lesion detection using dynamic shape features for diabetic retinopathy screening. IEEE Trans Med Imaging. 2016;35(4):1116–26. https://doi.org/10.1109/TMI.2015.2509785 (Epub 2015 Dec 17).
Silva PS, Cavallerano J, Haddad NMN, Tolls D, Thakore K, Patel B, Sehizadeh M, Tolson AM, Sun JK, Aiello LP. Comparison of Nondiabetic Retinal Findings Identified With Nonmydriatic Fundus Photography vs Ultrawide Field Imaging in an Ocular Telehealth Program. JAMA Ophthalmol. 2016;134(3):330–4. https://doi.org/10.1001/jamaophthalmol.2015.5605.
Fonda SJ, Bursell SE, Lewis DG, et al. The Indian Health Service primary care-based teleophthalmology program for diabetic eye disease surveillance and management. Telemed J E Health. 2020. https://doi.org/10.1089/tmj.2019.0281 (Online ahead of print).
Gu D, Agron S, May LN, et al. Nonmydriatic retinal diabetic screening in the primary care setting: assessing degree of retinopathy and incidence of nondiabetic ocular diagnoses. Telemed J E Health. 2020. https://doi.org/10.1089/tmj.2019 (Online ahead of print).
Toy BC, Aguinaldo T, Eliason J, Egbert J. Non-mydriatic fundus camera screening for referral-warranted diabetic retinopathy in a northern California safety-net setting. Ophthalmic Surg Lasers Imaging Retina. 2016;47(7):636–42. https://doi.org/10.3928/23258160-20160707-05.
Martin VY, Callirgos PGE, Martín MTM, et al. Satisfaction of patients and primary care professionals with a teleophthalmology-based screening programme for diabetic retinopathy in a rural area in Castilla y León, Spain. Rural Remote Health. 2020;20(1):5180. https://doi.org/10.22605/RRH5180 (Epub 2020 Jan 16).
Tsui I, Havunjian MA, Davis JA, Giaconi JA. Snapshot of teleretinal screening for diabetic retinopathy at the West Los Angeles Medical Center. Telemed J E Health. 2016;22(10):843–6. https://doi.org/10.1089/tmj.2015.0246 (Epub 2016 Mar 17).
Mastropasqua L, Perilli R, D’Aloisio R, et al. Why miss the chance? incidental findings while telescreening for diabetic retinopathy. Ohthalmic Epidemiol. 2020. https://doi.org/10.1080/09286586.2020.1715450 (Online ahead of print).
Turnin M-C, Schirr-Bonnans S, Chauchard M-C, Deglise P, Journot C, Lapeyre Y, Güell A, Lepage B, Hanaire H, Martini J. DIABSAT telemedicine itinerant screening of chronic complications of diabetes using a satellite. Telemed J E Health. 2017;23(5):397–403. https://doi.org/10.1089/tmj.2016.0185 (Epub 2016 Dec 21).
Strul S, Zheng Y, Gangaputra S, et al. Pediatric diabetic retinopathy telescreening. AAPOS. 2020;24(1):10.e1-10.e5. https://doi.org/10.1016/j.jaapos.2019.10.010 (Epub 2020 Jan 12).
Vujosevic S, Pucci P, Casciano M, Daniele AR, Bini S, Berton M, Cavarzeran F, Avogaro A, Lapolla A, Midena E. A decade-long telemedicine screening program for diabetic retinopathy in the North-East of Italy. J Diabetes Complications. 2017;31(8):1348–53. https://doi.org/10.1016/j.jdiacomp.2017.04.010 (Epub 2017 Apr 13).
Zapata MA, Arcos G, Fonollosa A, Abraldes M, Oleñik A, Gutierrez E, Garcia-Arumi J. Telemedicine for a general screening of retinal disease using nonmydriatic fundus cameras in optometry centers: three-year results. Telemed J E Health. 2017;23(1):30–6. https://doi.org/10.1089/tmj.2016.0020 (Epub 2016 May 26).
Clément M, et al. Evaluation of diabetic retinopathy screening using fundus photography: evaluation and epidemiologic factors at Nantes university medical center. J Fr Ophtalmol. 2019;42(3):281–7.
Porta M, Boscia F, Lanzetta P, Mannucci E, Menchini U, Simonelli F. Systematic screening of retinopathy in diabetes (REaD Project): an Italian Implementation Campaign. Eur J Ophthalmol. 2017;27(2):179–84. https://doi.org/10.5301/ejo.5000912 (Epub 2016 Nov 21).
Date RC, Shen KL, Shah BM, et al. Accuracy of detection and grading of diabetic retinopathy and diabetic macular edema using teleretinal screening. Ophthalmol Retina. 2019;3(4):343–9. https://doi.org/10.1016/j.oret.2018.12.003 (Epub 2018 Dec 24).
Pedrosa M, Silva JM, Silva JF, et al. SCREEN-DR: Collaborative platform for diabetic retinopathy. Int J Med Inf. 2018;120:137–46. https://doi.org/10.1016/j.ijmedinf.2018.10.005 (Epub 2018 Oct 18).
Sanborn GE, Wroblewski JJ. Evaluation of a combination digital retinal camera with spectral-domain optical coherence tomography (SD-OCT) that might be used for the screening of diabetic retinopathy with telemedicine: A pilot study. J Diabetes Complic. 2018;32(11):1046–50. https://doi.org/10.1016/j.jdiacomp.2018.08.010 (Epub 2018 Aug 11).
Conlin PR, Asefzadeh B, Pasquale LR, et al. Accuracy of a technology-assisted eye exam in evaluation of referable diabetic retinopathy and concomitant ocular diseases. Br J Ophthalmol. 2015;99(12):1622–7. https://doi.org/10.1136/bjophthalmol-2014-306536 (Epub 2015 May 20).
Walton OB 4th, Garoon RB, Weng CY, et al. Evaluation of automated teleretinal screening program for diabetic retinopathy. JAMA Ophthalmol. 2016;134(2):204–9. https://doi.org/10.1001/jamaophthalmol.2015.5083.
Bawankar P, Shanbhag N, Dhawan B, Palsule A, Kumar D, Chandel S, et al. Sensitivity and specificity of automated analysis of single-field non-mydriatic fundus photographs by Bosch DR Algorithm—Comparison with mydriatic fundus photography (ETDRS) for screening in undiagnosed diabetic retinopathy. PLoS ONE. 2017;12:e0189854.
Kanjee R, Dookeran RI, Mathen MK, Stockl FA, Leicht R. Six-year prevalence and incidence of diabetic retinopathy and cost-effectiveness of tele-ophthalmology in Manitoba. Can J Ophthalmol. 2017;52(Suppl 1):S15–8. https://doi.org/10.1016/j.jcjo.2017.09.022.
Horton MB, Brady CJ, Cavallerano J, et al. Practice guidelines for ocular telehealth-diabetic retinopathy, third edition. Telemed J E Health. 2020;26(4):495–543. https://doi.org/10.1089/tmj.2020.0006 (Epub 2020 Mar 25).
Park DW, Mansberger SL. Eye disease in patients with diabetes screened with telemedicine. Telemed J E Health. 2017;23(2):113–8. https://doi.org/10.1089/tmj.2016.0034 (Epub 2016 Jun 21).
Silva PS, Horton M, Clary D, Lewis DG, Sun JK, Cavallerano JD, Aiello LP. Identification of diabetic retinopathy and ungradable image rate with ultrawide field imaging in a national teleophthalmology. Program Ophthalmol. 2016;123(6):1360–7. https://doi.org/10.1016/j.ophtha.2016.01.043 (Epub 2016 Mar 2).
Bursell SE, Fonda SJ, Lewis DG, et al. Prevalence of diabetic retinopathy and diabetic macular edema in a primary care-based teleophthalmology program for American Indians and Alaskan Natives. PLoS ONE. 2018;13(6):e0198551. https://doi.org/10.1371/journal.pone.0198551 (eCollection 2018).
Mansberger SL, Sheppler C, Barker G, et al. Long-term comparative effectiveness of telemedicine in providing diabetic retinopathy screening examinations: a randomized clinical trial. JAMA Ophthalmol. 2015;133(5):518–25. https://doi.org/10.1001/jamaophthalmol.2015.1.
Ramakrishnan R, Khadar AMA, Srinivasan K, et al. Diabetes mellitus in the Tamil Nadu State-noncommunicable diseases nurse model in diabetic retinopathy screening. Indian J Ophthalmol. 2020;68(Suppl 1):S78–82. https://doi.org/10.4103/ijo.IJO_1987_19.
Afshar AR, Oldenburg CE, Stewart JM. A novel hybrid fixed and mobile ultra-widefield imaging program for diabetic teleretinopathy screening. Ophthalmol Retina. 2019;3(7):576–9. https://doi.org/10.1016/j.oret.2019.03.007 (Epub 2019 Mar 20).
Kern C, Kortuem K, Hamilton R, et al. Clinical outcomes of a hospital-based teleophthalmology service: what happens to patients in a virtual clinic? Ophthalmol Retina. 2019;3(5):422–8. https://doi.org/10.1016/j.oret.2019.01.011 (Epub 2019 Jan 21).
Hussain N, Edraki M, Tahhan R, et al. Telemedicine for diabetic retinopathy screening using an ultra-widefield fundus camera. Clin Ophthalmol. 2017;14(11):1477–82. https://doi.org/10.2147/OPTH.S135287 (eCollection 2017).
Bartnik SE, Copeland SP, Aicken AJ, et al. Optometry-facilitated teleophthalmology: an audit of the first year in Western Australia. Clin Exp Optom. 2018;101(5):700–3. https://doi.org/10.1111/cxo.12658 (Epub 2018 Feb 14).
Alam M, Le D, Lim JI, Chan RVP, Yao X. Supervised machine learning based multi-task artificial intelligence classification of retinopathies. J Clin Med. 2019;8(6):872. https://doi.org/10.3390/jcm8060872
Grau E, Horn F, Nixdorff U, Michelson G. OCT and IOP Findings in a healthy worker cohort: results from a teleophthalmic study in occupational medicine. Graefes Arch Clin Exp Ophthalmol. 2019;257(11):2571–8. https://doi.org/10.1007/s00417-019-04457-1 (Epub 2019 Sep 5).
Kortuem K, Fasler K, Charnley A, et al. Implementation of medical retina virtual clinics in a tertiary eye care referral centre. Br J Ophthalmol. 2018;102(10):1391–5. https://doi.org/10.1136/bjophthalmol-2017-311494 (Epub 2018 Jan 6).
Wang YT, Tadarati M, Wolfson Y, et al. Comparison of prevalence of diabetic macular edema based on monocular fundus photography vs optical coherence tomography. JAMA Ophthalmol. 2016;134(2):222–8. https://doi.org/10.1001/jamaophthalmol.2015.5332.
Gadkari S, MRCOphth FRCS. Innovative model for telemedicine-based screening for diabetic retinopathy in the developing world. Can J Opthamol. 2016;51(3):e109–11. https://doi.org/10.1016/j.jcjo.2015.11.010 (50TH ANNIVERSARY ISSUE).
Taraprasad Das and Rajeev Reddy Pappuru. Telemedicine in diabetic retinopathy: access to rural India. Indian J Ophthalmol. 2016;64(1):84–6.
Matimba A, Woodward R, Tambo E, Ramsay M, Gwanzura L, Guramatunhu S. Tele-ophthalmology: opportunities for improving diabetes eye care in resource- and specialist-limited Sub-Saharan African Countries. J Telemed Telecare. 2016;22(5):311–6. https://doi.org/10.1177/1357633X15604083 (Epub 2015 Sep 24).
Toy BC, Myung DJ, He L, Pan CK, Chang RT, Polkinhorne A, Merrell D, Foster D, Blumenkranz MS. Smartphone- based dilated fundus Photography and near visual acuity testing as inexpensive screening tools detect referral warranted diabetic eye disease. Retina. 2016;36(5):1000–8. https://doi.org/10.1097/IAE.0000000000000955.
Martinez JA, Parikh PD, Wong RW, et al. Telemedicine for diabetic retinopathy screening in an urban, insured population using fundus cameras in a primary care office setting. Ophthalmic Surg Lasers Imaging Retina. 2019;50(11):e274–7. https://doi.org/10.3928/23258160-20191031-14.
Keshvardoost S, Bahaadinibeigy K, Shadman H, et al. Design, development, and evaluation of a teleophthalmology system using a low-cost fundus camera. Acta Inform Med. 2020;28(1):12–7. https://doi.org/10.5455/aim.2019.28.12-17.
Zhang W, Nicholas P, Schuman SG, et al. Screening for diabetic retinopathy using a portable, noncontact, nonmydriatic handheld retinal camera. J Diabetes Sci Technol. 2017;11(1):128–34. https://doi.org/10.1177/1932296816658902 (Epub 2016 Jul 11).
Bastawrous A. Smartphone fundoscopy. Ophthalmology. 2012;119(2):432–3.
Micheletti M, Hendrick AM, Khan FN, Ziemer D, Pasquel FJ. Current and next generation portable screening devices for diabetic retinopathy. J Diabetes Sci Technol. 2016;10(2):295–300. https://doi.org/10.1177/1932296816629158 (Published online 2016 Feb 16).
Choon HT, Bhone MK, Helen S, Colin ST, Lorainne TC. Use of smartphones to detect diabetic retinopathy: scoping review and meta-analysis of diagnostic test accuracy studies. J Med Internet Res. 2020;22(5):e16658. https://doi.org/10.2196/16658 (Published online 2020 May 15).
Kim TN, Meyers F, Reber C, et al. A smartphone-based tool for rapid, portable, and automated wide-field retinal imaging. Transl Vis Sci Technol. 2018;7(5):21.
Bhat S, Bhaskaranand M, Ramachandra C, Margolis TP, Fletcher DA, Solanki K. Fully-automated diabetic retinopathy screening using cellphone-based cameras. Invest Ophthalmol Vis Sci. 2015;56(7):1428–1428.
Bolster NM, Giardini ME, Bastawrous A. The diabetic retinopathy screening workflow potential for smartphone imaging. J Diabetes Sci Technol. 2016;10(2):318–24. https://doi.org/10.1177/1932296815617969 (Published online 2015 Nov 23).
Sean C, David C, Geoffrey T, Karen H, David M, Suman T. Utility and feasibility of teleophthalmology using a smartphone-based ophthalmic camera in screening camps in Nepal. Asia Pac J Ophthalmol (Phila). 2020;9(1):54–8. https://doi.org/10.1097/01.APO.0000617936.16124 (Published online 2020 Feb 3).
Bastawrous A, Giardini ME, Bolster NM, Peto T, Shah N, Livingstone I, Weiss HA, Hu S, Rono H, Kuper H, Burton M. Clinical validation of smartphone based adapter: peek retina for optic disc imaging in Kenya. JAMA Ophthalmol. 2016;134(2):151–8. https://doi.org/10.1001/jamaophthalmol.2015.4625 (Author manuscript; available in PMC 2017 Feb 22. Published in final edited form as: JAMA Ophthalmol).
Patel TP, Kim NT, Yu G, Dedania VS, Lieu P, Qian CX, Besirli CG, Demirci H, Margolis T, Fletcher DA, Paulus YM. Smartphone-based, rapid, wide-field fundus photography for diagnosis of pediatric retinal diseases. Transl Vis Sci Technol. 2019;8(3):29. https://doi.org/10.1167/tvst.8.3.29.
Lekha T, Ramesh S, Sharma A, Abinaya G. MII RetCam assisted smartphone based fundus imaging for retinopathy of prematurity. Indian J Ophthalmol. 2019;67(6):834–9. https://doi.org/10.4103/ijo.IJO_268_19.
Wintergerst MW, Jansen LG, Holz F, Finger RP. a novel device for smartphone-based fundus imaging and documentation in clinical practice: comparative image analysis study. JMIR Mhealth Uhealth. 2020;8(7):e17480. https://doi.org/10.2196/17480 (Published online 2020 Jul 29).
Khanamiri NH, Nakatsuka A, El-Annan J. Smartphone fundus photography. J Vis Exp. 2017;125:55958. https://doi.org/10.3791/55958 (Published online 2017 Jul 6).
Kim Y, Chao DL. Comparison of smartphone ophthalmoscopy vs conventional direct ophthalmoscopy as a teaching tool for medical students: the COSMOS study. Clin Ophthalmol. 2019;13:391–401. https://doi.org/10.2147/OPTH.S190922 (Published online 2019 Feb 18).
Saeed E, Szymkowski M, Saeed K, et al. An approach to automatic hard exudate detection in retina color images by a telemedicine system based on the d-eye sensor and image processing algorithms. Sensors (Basel). 2019;19(3):695. https://doi.org/10.3390/s19030695.
Kozak I, Payne JF, Schatz P, Al-Kahtani E, Winkler M. Teleophthalmology image-based navigated retinal laser therapy for diabetic macular edema: a concept of retinal telephotocoagulation. Graefes Arch Clin Exp Ophthalmol. 2017;255(8):1509–13. https://doi.org/10.1007/s00417-017-3674-1 (Epub 2017 Apr 26).
Nguyen H, Tan GSW, Tapp RJ, Mital S, Ting SWD, Wong HT, Tan C, Laude A, Tai ES, Chuan Tan N, Finkelstein E, Wong TY, Lamoureux EL. 10 cost-effectiveness of a national telemedicine diabetic retinopathy screening program in Singapore. Ophthalmology. 2016;123(12):2571–80. https://doi.org/10.1016/j.ophtha.2016.08.021 (Epub 2016 Oct 7).
Abràmoff MD, Leng T, Ting DSW, Rhee K, Horton MB, Brady CJ, Chiang MF. Automated and computer-assisted detection, classification, and diagnosis of diabetic retinopathy. Telemed e-Health. 2020;26(4):544–50. https://doi.org/10.1089/tmj.2020.0008.
Rodríguez Villa S, Suárez Muñiz MT, Valle DDDR, et al. Enferm Retinopathy diabetic screening by non-mydriatic retinography: concordance between primary care physicians, nurses and ophthalmologists. Clin. 2018;28(1):44–8. https://doi.org/10.1016/j.enfcli.2017.04.010 (Epub 2017 Jun 16).
Boucher MC, Nguyen MTD, Qian J. Assessment of training outcomes of nurse readers for diabetic retinopathy telescreening: validation study. JMIR Diabetes. 2020;5(2):e17309. https://doi.org/10.2196/17309.
Abràmoff MD, Lou Y, Erginay A, Clarida W, Amelon R, Folk JC, et al. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Invest Ophthalmol Vis Sci. 2016;57:5200–6.
Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. npj Digit Med. 2018;1:39.
Abràmoff MD, Folk JC, Han DP, Walker JD, Williams DF, Russell SR, et al. Automated analysis of retinal images for detection of referable diabetic retinopathy. JAMA Ophthalmol. 2013;131:351–7.
US Food and Drug Administration. FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems. News Release, US Food and Drug Administration; April 2018.
Ribeiro L, Oliveira CM, Neves C, Ramos JD, Ferreira H, Cunha-Vaz J. Screening for diabetic retinopathy in the central region of Portugal. Added value of automated ‘disease/no disease’ grading. Ophthalmologica. 2015;233:96–103.
Ribeiro ML, Nunes SG, Cunha-Vaz JG. Microaneurysm turnover at the macula predicts risk of development of clinically significant macular edema in persons with mild nonproliferative diabetic retinopathy. Diabetes Care. 2012;36:1254–9.
Ting DSW, Cheung CY-L, Lim G, Tan GSW, Quang ND, Gan A, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318:2211–23.
Larsen N, Godt J, Grunkin M, Lund-Andersen H, Larsen M. Automated detection of diabetic retinopathy in a fundus photographic screening population. Invest Ophthalmol Vis Sci. 2003;44:767–71.
Hansen AB, Hartvig NV, Jensen MS, Borch-Johnsen K, Lund- Andersen H, Larsen M. Diabetic retinopathy screening using digital non-mydriatic fundus photography and automated image analysis. Acta Ophthalmol Scand. 2004;82:666–72.
Larsen M, Godt J, Larsen N, Lund-Andersen H, Sjølie AK, Agardh E, et al. Automated detection of fundus photographic red lesions in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2003;44:761–6.
Gargeya R, Leng T. Automated identification of diabetic retinopathy using deep learning. Ophthalmology. 2017;124:962–9.
Li Z, Keel S, Liu C, He Y, Meng W, Scheetz J, et al. An automated grading system for detection of vision-threatening referable diabetic retinopathy on the basis of color fundus photographs. Diabetes Care. 2018;41:2509–16.
Stevenson CH, Hong SC, Ogbuehi KC. Development of an artificial intelligence system to classify pathology and clinical features on retinal fundus images. Clin Exp Ophthalmol. 2019;47(4):484–9. https://doi.org/10.1111/ceo.13433 (Epub 2018 Nov 15).
Das V, Dandapat S, Bora PK. A novel diagnostic information based framework for super-resolution of retinal fundus images. Comput Med Imaging Graph. 2019;72:22–33. https://doi.org/10.1016/j.compmedimag.2019.01.002 (Epub 2019 Feb 1).
Zhu X, Xu Y, Lu L, Zou H, et al. Patients’ perspectives on the barriers to referral after telescreening for diabetic retinopathy in communities. BMJ Open Diabetes Res Care. 2020;8(1):e000970. https://doi.org/10.1136/bmjdrc-2019-000970.
Liu, Zupan NJ, Swearingen R, Jacobson N, Carlson JN, Mahoney JE, Klein R, Bjelland TD, Smith MA. Identification of barriers, facilitators and system-based implementation strategies to increase teleophthalmology use for diabetic eye screening in a rural US primary care clinic: a qualitative study. http://orcid.org/0000-0002-0700-0148Yao.
Gibson MD. Estimates of the percentage of US adults with diabetes who could be screened for diabetic retinopathy in primary care settings. JAMA Ophthalmol. 2019;137(4):440–4. https://doi.org/10.1001/jamaophthalmol.2018.6909 (Published online 2019 Jan 31).
Valikodath NG, Leveque TK, Wang SY, et al. Patient attitudes toward telemedicine for diabetic retinopathy. Telemed J E Health. 2017;23(3):205–12. https://doi.org/10.1089/tmj.2016.0108 (Epub 2016 Jun 23).
Teo ZL, Tham YC, Yu M, et al. Do we have enough ophthalmologists to manage vision-threatening diabetic retinopathy? A global perspective. Eye (Lond). 2020;34(7):1255–61. https://doi.org/10.1038/s41433-020-0776-5 (Epub 2020 Jan 28).
Mehlsen J, Erlandsen M, Poulsen PL, Bek T. Individualized optimization of the screening interval for diabetic retinopathy: a new model. Acta Ophthalmol. 2012;90:109–14.
Kirkizlar E, Serban N, Sisson JA, Swann JL, Barnes CS, Williams MD. Evaluation of telemedicine for screening of diabetic retinopathy in the veterans health administration. Ophthalmology. 2013;120(12):2604–10.
Taylor CR, Merin LM, Salunga AM, Hepworth JT, Crutcher TD, O’Day DM, Pilon BA. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: the Vine Hill study. Diabetes Care. 2007;30(3):574–8.
Cuadros J, Bresnick G. EyePACS: an adaptable telemedicine system fordiabetic retinopathy screening. J Diabetes Sci Technol. 2009;3(3):509–16.
Scanlon PH. The English National Screening Programme for diabetic retinopathy 2003–2016. Acta Diabetol. 2017;54:515–25.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Janusz Pieczyński: PubMed search, analysis of articles, manuscript design and preparation, drafting of the manuscript. Patrycja Kuklo: PubMed search, analysis of articles, manuscript preparation. Andrzej Grzybowski: study concept, design and supervision, drafting of the manuscript.
Disclosures
Janusz Pieczyński, Patrycja Kuklo and Andrzej Grzybowski have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The authors declare that there is no conflict of interest regarding the publication of this paper.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Pieczynski, J., Kuklo, P. & Grzybowski, A. The Role of Telemedicine, In-Home Testing and Artificial Intelligence to Alleviate an Increasingly Burdened Healthcare System: Diabetic Retinopathy. Ophthalmol Ther 10, 445–464 (2021). https://doi.org/10.1007/s40123-021-00353-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-021-00353-2