Abstract
Introduction
The transition of acute to chronic postoperative pain (CPP) remains a significant burden to the rehabilitation of patients. The research for adjuvants to prevent CPP continues; among others, dexmedetomidine and lidocaine seem promising agents.
Methods
This is a long-term follow-up of a randomized, placebo-controlled, double-blind study on women who underwent open abdominal gynecological surgery and received dexmedetomidine or lidocaine or placebo infusion perioperatively (n = 81). The effect of these adjuvants on the development of CPP and neuropathic pain was assessed during a 12-month follow-up. Eighty-one (81) women ASA I–II, aged between 30 and 70 years, were randomly assigned to receive either dexmedetomidine (DEX group) or lidocaine (LIDO group) or placebo (CONTROL group) perioperatively. Before anesthesia induction, all patients received a loading intravenous dose of either 0.6 μg/kg dexmedetomidine or 1.5 mg/kg lidocaine or placebo, followed by 0.6 μg/kg/h dexmedetomidine or 1.5 mg/kg/h lidocaine or placebo until last suture. Patients were followed up to obtain the long-term outcomes at 3, 6, and 12 months. At these time-points, pain intensity was assessed with the Numerical Rating Scale, (NRS: 0–10) and the development of neuropathic elements with the Douleur Neuropathique 4 (DN4) score. Prognostic parameters that could affect chronic pain and its components were also identified.
Results
Data from 74 women were analyzed. Dexmedetomidine significantly reduced NRS scores comparing to placebo at 3 months (p = 0.018), while at 6 months, lidocaine was found superior to placebo (p = 0.02), but not to dexmedetomidine, in preventing neuropathic pain (DN4 < 4). Regarding secondary endpoints, higher NRS cough scores at 48 h were associated with statistically significant NRS and DN4 scores at 3, 6, and 12 months (p < 0.02). At 6 months, a statistically significant correlation was also found between higher NRS values and older age (p = 0.020).
Conclusions
Dexmedetomidine was superior to placebo regarding the duration and severity of CPP, while lidocaine exhibited a protective effect against neuropathic elements of CPP.
Trial registration
ClinicalTrials.gov identifier, NCT03363425.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The transition of acute to chronic postoperative pain remains a significant problem and there is ongoing research for adjuvants that could possibly prevent it. |
We present the long-term follow-up outcomes (chronic pain/neuropathic pain) of an RCT with primary endpoint the effect of lidocaine and dexmedetomidine on acute postoperative pain after open abdominal gynecological surgery. |
Dexmedetomidine significantly reduced pain scores compared to placebo at 3 months, while lidocaine was found superior to placebo in preventing neuropathic pain (Douleur Neuropathique score, DN4 < 4) at 6 months. |
Increased NRS scores at 48 led to statistically significant chronic pain NRS scores at 3, 6, and 12 months. |
The advanced age of patients was also identified to lead to increased chronic pain NRS scores at 6 months after surgery. |
Introduction
The term chronic postoperative pain (CPP) is used to describe the pain that develops after a surgical procedure and persists beyond the healing process i.e., at least 3 months after surgery [1]. CPP is an important clinical problem that may impair patients’ physical function and reduce the quality of their lives; therefore, its early prevention during the perioperative period is crucial [2]. Myomectomy and hysterectomy are common gynecological surgical procedures performed for various indications such as pain, menorrhagia, or dysmenorrhea [3]. According to the literature, the incidence of chronic pain after hysterectomy is reported to be 5–50% [4,5,6], while prospective studies of hysterectomy for benign conditions suggest that up to a quarter of the patients still report pain 1 year after the operation [7, 8].
Even though the transition of acute postoperative to chronic pain has been extensively investigated in the past years and the quality of studies has improved, CPP still remains an unsolved issue [9]. The identification of risk factors such as young age, female sex, psychological disorders, preoperative painful syndromes or severe acute postoperative pain, surgical factors and extent of injury, would help in the reduction of CPP incidence [10, 11].
The anesthetic technique has been proposed as an important factor that could reduce the incidence of CPP [12], and there is ongoing research for adjuvants that could be beneficial. Among other agents, dexmedetomidine, a highly selective α2 adrenoreceptor agonist, and lidocaine, a well-established local anesthetic, seem promising. The existing literature on the effects of dexmedetomidine versus lidocaine on CPP and neuropathic elements is very limited. Dexmedetomidine has shown positive effects on acute postoperative pain and opioid consumption [13, 14] but it has been very little investigated in the clinical setting for CPP [15,16,17]. Intraoperatively, it has been given as an intravenous (iv) loading dose of 0.5–1 μg/kg over 10 min, followed by an iv infusion of 0.2–0.7 μg/kg/h [18]. Lidocaine has been more extensively investigated in the chronic pain clinical setting [19, 20]. It exerts its effects through sodium channel blockade [21], inhibition of G proteins [22], and NMDA receptors [23]. In patients undergoing open abdominal surgeries, lidocaine has been given as a bolus iv dose of 1.5–2 mg/kg prior to induction/incision, followed by an infusion of 1.5–3 mg/kg/h [24,25,26].
We performed a long-term (1-year) follow-up to evaluate the effects of intraoperative iv infusion of dexmedetomidine and lidocaine on the occurrence of chronic pain after abdominal gynecological surgery. The study endpoints were the development of CPP with or without neuropathic elements at 3, 6, and 12 months postoperatively. We also identified possible risk factors for the transition of acute postoperative pain to CPP. These long-term outcomes were secondary endpoints of a randomized, double-blind study investigating the effect of dexmedetomidine and lidocaine on acute postoperative pain and analgesic consumption [27].
Methods
The key features of the original trial [27], including design, setting, eligibility, interventions, outcome measures, and sample size calculation are summarized in Table 1. Patients were contacted at 3, 6, and 12 months postoperatively by telephone for a brief interview to assess any residual pain or uncomfortable sensation. The researcher that conducted the telephone interviews was blinded regarding the intervention group of each patient. First, the patients were asked if they had any residual pain in the surgical area or around the surgical incision. If they answered “yes”, then they were asked to provide further information regarding pain site and pain intensity using a numerical pain scale (NRS) from 0 (no pain) to 10 (the worst pain imaginable). To identify possible neuropathic characteristics of the pain, the participants were asked the ten questions from the Greek version of the DN4 [28]. We adapted the questionnaire for telephone interview (Table 2) according to a previous study [29]. The collection of the chronic pain data was completed in January 2021.
Statistical Analysis
Means and standard deviations were used to describe all scale measurements such as age, NRS, or the DN4 scale. Categorical variables were described with the use of counts and percentages. Repeated measures general linear models examined the differences, in NRS and DN4 scales, observed across time and between the three study groups, adjusting for the effect of age, surgery duration, and the NRS scores during the first 48 h following the surgery and the Bonferroni correction was applied to adjust for multiple comparisons. To identify parameters that could affect the DN4 scores, a repeated measures model was applied, which included the duration of the surgery, the age of the patients and the NRS cough scores measured at 48 h after surgery. Firth’s penalized logistic regression was used to assess the differences in the odds of having neuropathic pain at 6 months after surgery, depending on the study group [30, 31]. Statistical significance was equal to 0.05 in all cases, including the analyses carried out under the Bonferroni correction. All analyses were conducted with the use of STATISTICA v12.0, except for the penalized logistic regression that was carried out on R.
Results
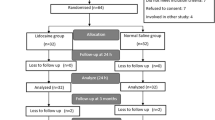
Data from 74 women (24 patients in the DEX group, 25 in the LIDO group and 25 in the Control group) were included in chronic pain analysis (see attached Flowchart). Seven patients were lost to follow-up as we were unable to contact them on the given telephone number in any of the three occasions (3, 6, or 12 months) after three attempts. Patient’s characteristics did not differ among the three groups (p > 0.05) (Table 3). Also, there was no statistical difference regarding the type of surgery (p = 0.962) among the groups. The surgical team included four senior surgeons with at least 15 years of experience.
At 3 months, the DEX group showed statistically significant lower pain scores compared to CONTROL group (p = 0.018). No other statistically differences were observed regarding pain scores at 3, 6, and 12 months among the groups (Table 4).
The repeated-measures model that was applied at each time point to identify parameters that could affect the outcomes included the NRS scores (rest and cough), the duration of the surgery, as well as the age of the patients. The analysis showed that the cough NRS scores at 48 h had a statistically significant effect on the chronic pain NRS scores (Table 5). This correlation was revealed by the repeated measures general linear model (GLM) at all three times (Table 5; Fig. 1). Higher values of NRS pain scores at all time periods are therefore expected for higher values of NRS cough pain scores at the 48-h measurement.
A statistically significant relation (p = 0.020) was also found between advanced age of patients and higher values of chronic pain NRS scores at 6 months after surgery. This relationship was borderline nonsignificant at 3 and 12 months with p values equal to 0.079 and 0.092, respectively.
Analysis for the DN4 Scores
The DN4 scores across the three time points for each of the treatment groups are shown in Table 6. Even though the control group appeared to score higher at all periods compared to the other two groups (Fig. 2), these differences were not statistically significant (p > 0.05) (Table 6).
In our study, although many patients reported neuropathic elements, most of them did not fulfil the literature cut-off score of 4 for their pain to be diagnosed as neuropathic [32]. Therefore, at all time points, the percentage of patients suffering from neuropathic pain was relatively small, as shown in Table 7. Fisher's exact test showed a statistically significant difference at 6 months (p = 0.031).
The comparative bar chart in Fig. 3 shows that more patients are expected to suffer with neuropathic pain in the control group. The penalized logistic regression model also showed a statistically significant difference in the neuropathic pain depending on the treatment group (p = 0.048). This difference was observed between the LIDO (0%) and CONTROL (20%) group (p = 0.02), but not among the other groups [DEX (4.2%) vs. CONTROL (p = 0.106) and DEX vs. LIDO (p = 0.442)].
The analysis showed that the 48-h cough NRS scores had a statistically significant effect on the DN4 scores at all three times, thus 3, 6, and 12 months, with p values equal to 0.01, < 0.01, and < 0.01, respectively. Higher values of DN4 at all time periods are therefore expected for higher values of NRS cough scores at 48 h, which are depicted in Table 8.
Adverse Effects of the Investigated Agents
Intraoperative hypotension and bradycardia were more often observed in the study groups (DEX and LIDO) compared to the CONTROL group, but the differences were not statistically significant (p = 0.357 and p = 0.566, respectively). A patient of the LIDO group developed ventricular ectopic beats, which resolved after discontinuation of the intervention.
No other adverse effects were reported during the postoperative period.
Discussion
Our results showed that perioperative iv infusion of dexmedetomidine comparing to placebo had a beneficial effect on the prevention of CPP at 3 months, while lidocaine infusion prevented the development of neuropathic pain at 6 months after gynecological surgery. To our knowledge, this is the first study comparing intraoperative dexmedetomidine and lidocaine with placebo with regards to CPP development. Our long-term follow-up showed superiority of the studied drugs over placebo and highlighted predisposing factors for development of chronic pain with or without neuropathic elements.
The first finding, that dexmedetomidine at 3 months prevented the development of CPP compared to placebo, is also supported by one study, which investigated perioperative infusion of dexmedetomidine in patients undergoing breast cancer surgery [15]. However, in that study, patients had received much greater doses of the investigated drug (1 μg/kg iv bolus, followed by a continuous infusion of 0.5 μg/kg/h iv till the completion of surgery, and then the dose was tapered to 0.2 μg/kg/h for up to 24 h). The beneficial effect of dexmedetomidine has also been shown in an experimental study, where it attenuated persistent postsurgical pain by upregulating K+–Cl− cotransporter-2 in the spinal dorsal horn in rats [33].
The second finding of our study was that lidocaine proved to be superior to placebo regarding DN4 scores, making it a useful drug for the prevention of postsurgical neuropathic pain. For the assessment of neuropathic elements of pain, we used the DN4 questionnaire, a validated and widely used screening tool for the identification and classification of neuropathic pain [32]. It has been shown to achieve an 83% sensitivity and 90% specificity when compared to clinical diagnosis [34]. A former study had successfully performed the DN4 neuropathic pain questionnaire over the telephone to identify possible neuropathic elements [29]. In this study [29], for the first seven symptom items, the respondents answered “yes” or “no” to whether their pain could be described as burning, painful cold, electric shocks, tingling, pins and needles, numbness, and itching. The patients were also asked if their pain area was sensitive to touch, sensitive to pin prick, and sensitive to light brushing, which resembles the clinical examination. A score of 1 was given for each “yes” answer and 0 for each “no” answer. A score of < 4 suggested that the pain was unlikely to be neuropathic. This important finding might be supported by the fact that lidocaine is already a very useful drug, used as infusion in chronic neuropathic pain states, as extensively analyzed in a recent review [35] and in a metanalysis [36].
Moreover, our results showed that the pain scores (NRS cough) at 48 h had a statistically significant effect on the chronic pain scores at all three times, making acute postoperative pain an indication of transition to chronic pain. Fassoulaki et al. have demonstrated that pain during the immediate postoperative period can predict chronic pain after breast surgery for cancer [37]. However, in this study patients who developed chronic pain had experienced higher pain intensity at rest during the first nine postoperative hours rather than pain at cough at 48 h as in our study. Other studies also support our finding [38, 39].
Pain intensity at 48 h postoperatively was found to be a significant risk factor for the development of neuropathic pain, as higher DN4 scores at 3, 6, and 12 months were reported from patients with higher NRS cough scores at 48 h. This finding has been also identified by other studies that have highlighted the role of acute pain as a risk factor for the development of long-term neuropathic pain [40, 41].
Age may also play an important role in the development of CPP. We found that at 6 months after surgery, older patients suffered more severe pain. This is not in agreement with previous findings, as Martinez et al. have suggested the opposite, i.e., that younger age is a predictive factor for chronic pain [42]. Also, according to Schug and Bruce, younger age seems to be in most studies a relatively consistent demographic risk factor for CPP in adults [11].
Limitations and Strengths
This long-term study on CPP derives from a RCT that was powered to reveal differences in acute postoperative pain; thus, the sample size may be relatively small for chronic pain outcomes. Another possible limitation is that we did not study different doses of the administered drugs. Also, several possible risk factors of CPP, such as gender, preexisting pain, and psychological factors were not investigated in the present study, as it was designed to minimize these confounding factors; the studied population were all females (ASA I and II with no major comorbidities or prior disability), while patients with preexisting pain or analgesic consumption, or those treated for depression and anxiety were excluded according to protocol (Table 1). Additionally, the duration of surgery did not differ among the groups (Table 3) and the patients with surgical complications were excluded from follow-up and analysis. We did not assess other possible predisposing factors for CPP, such as education level and lifestyle.
A strength of this study is that the patients were followed up for quite a long time (12 months) and also that we managed to have a high percentage of patients (74/81 or 91.35%) that completed the follow-up period. This is important since previous similar studies report significant dropout rates, ranging from approximately 34% [43] to 43.6% after 12 months [44].
Conclusions
The present study demonstrated that perioperative infusion of dexmedetomidine or lidocaine may exert a beneficial effect on the development and characteristics of CPP after abdominal gynecological surgery. Dexmedetomidine proved to be superior to placebo regarding the duration and severity of CPP, while lidocaine exhibited a protective effect against neuropathic elements of CPP over placebo. We consider that the findings of this RCT could provide the foundation for future studies.
References
Treede RD, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003–7.
Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017;10:2287–98.
Parker WH. Uterine myomas: management. Fertil Steril. 2007;88(2):255–71.
Brandsborg B, et al. Chronic pain after hysterectomy. Acta Anaesthesiol Scand. 2008;52(3):327–31.
Han C, et al. Incidence and risk factors of chronic pain following hysterectomy among Southern Jiangsu Chinese Women. BMC Anesthesiol. 2017;17(1):103.
Pinto PR, et al. Risk factors for persistent postsurgical pain in women undergoing hysterectomy due to benign causes: a prospective predictive study. J Pain. 2012;13(11):1045–57.
Gimbel H, et al. Randomised controlled trial of total compared with subtotal hysterectomy with one-year follow up results. BJOG. 2003;110(12):1088–98.
Humalajärvi N, et al. Quality of life and pelvic floor dysfunction symptoms after hysterectomy with or without pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol. 2014;182:16–21.
Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008;101(1):77–86.
Lavand’homme P. Transition from acute to chronic pain after surgery. Pain. 2017;158(Suppl 1):S50-s54.
Schug SA, Bruce J. Risk stratification for the development of chronic postsurgical pain. Pain Rep. 2017;2(6): e627.
Searle R, Simpson K. Chronic post-surgical pain. Contin Educ Anaesth Crit Care Pain. 2009;10(1):12–4.
Jessen Lundorf L, Korvenius Nedergaard H, Møller AM. Perioperative dexmedetomidine for acute pain after abdominal surgery in adults. Cochrane Database Syst Rev. 2016;2: CD010358.
Galvin IM, et al. Pharmacological interventions for the prevention of acute postoperative pain in adults following brain surgery. Cochrane Database Syst Rev. 2019;2019(11): CD011931.
Jain G, et al. Effect of the perioperative infusion of dexmedetomidine on chronic pain after breast surgery. Indian J Palliat Care. 2012;18(1):45–51.
Liu Y, et al. Dexmedetomidine relieves neuropathic pain in rats with chronic constriction injury via the Keap1-Nrf2 pathway. Front Cell Dev Biol. 2021;9: 714996.
Wang X, Liu Q. Dexmedetomidine relieved neuropathic pain and inflammation response induced by CCI through HMGB1/TLR4/NF-κB signal pathway. Biol Pharm Bull. 2021. https://doi.org/10.1248/bpb.b21-00329.
Dholakia C, et al. The impact of perioperative dexmedetomidine infusion on postoperative narcotic use and duration of stay after laparoscopic bariatric surgery. J Gastrointest Surg. 2007;11(11):1556–9.
Bailey M, et al. Perioperative lidocaine infusions for the prevention of chronic postsurgical pain: a systematic review and meta-analysis of efficacy and safety. Pain. 2018;159(9):1696–704.
Kendall MC, et al. The effect of intraoperative systemic lidocaine on postoperative persistent pain using initiative on methods, measurement, and pain assessment in clinical trials criteria assessment following breast cancer surgery: a randomized, double-blind placebo-controlled trial. Pain Pract. 2018;18(3):350–9.
Hollmann MW, Durieux ME. Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology. 2000;93(3):858–75.
Hollmann MW, et al. Local anesthetic inhibition of G protein-coupled receptor signaling by interference with Galpha(q) protein function. Mol Pharmacol. 2001;59(2):294–301.
Sugimoto M, Uchida I, Mashimo T. Local anaesthetics have different mechanisms and sites of action at the recombinant N-methyl-d-aspartate (NMDA) receptors. Br J Pharmacol. 2003;138(5):876–82.
Toner AJ, et al. A pilot multicentre randomised controlled trial of lidocaine infusion in women undergoing breast cancer surgery. Anaesthesia. 2021;76(10):1326–41.
Herroeder S, et al. Systemic lidocaine shortens length of hospital stay after colorectal surgery: a double-blinded, randomized, placebo-controlled trial. Ann Surg. 2007;246(2):192–200.
Koppert W, et al. Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery. Anesth Analg. 2004;98(4):1050–5.
Rekatsina M, Theodosopoulou P, Staikou C. Effects of intravenous dexmedetomidine versus lidocaine on postoperative pain, analgesic consumption and functional recovery after abdominal gynecological surgery: a randomized placebo-controlled double blind study. Pain Physician. 2021;24(7):E997-e1006.
Sykioti P, et al. Validation of the Greek version of the DN4 diagnostic questionnaire for neuropathic pain. Pain Pract. 2015;15(7):627–32.
Zghoul N, et al. Prevalence of chronic pain with neuropathic characteristics: a randomized telephone survey among medical center patients in Kuwait. J Pain Res. 2017;10:679–87.
Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80(1):27–38.
Wang X. Firth logistic regression for rare variant association tests. Front Genet. 2014;5:187.
Bouhassira D, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114(1–2):29–36.
Dai S, et al. Dexmedetomidine attenuates persistent postsurgical pain by upregulating K(+)–Cl(−) cotransporter-2 in the spinal dorsal horn in rats. J Pain Res. 2018;11:993–1004.
Harifi G, et al. Validity and reliability of the Arabic adapted version of the DN4 questionnaire (Douleur Neuropathique 4 Questions) for differential diagnosis of pain syndromes with a neuropathic or somatic component. Pain Pract. 2011;11(2):139–47.
Kandil E, Melikman E, Adinoff B. Lidocaine infusion: a promising therapeutic approach for chronic pain. J Anesth Clin Res. 2017;8(1):697.
Zhu B, et al. Intra-venous lidocaine to relieve neuropathic pain: a systematic review and meta-analysis. Front Neurol. 2019;10:954–954.
Fassoulaki A, et al. Acute postoperative pain predicts chronic pain and long-term analgesic requirements after breast surgery for cancer. Acta Anaesthesiol Belg. 2008;59(4):241–8.
Shipton E. Post-surgical neuropathic pain. ANZ J Surg. 2008;78(7):548–55.
Richebé P, Capdevila X, Rivat C. Persistent postsurgical pain: pathophysiology and preventative pharmacologic considerations. Anesthesiology. 2018;129(3):590–607.
Lavand’homme PM, et al. Pain trajectories identify patients at risk of persistent pain after knee arthroplasty: an observational study. Clin Orthop Relat Res. 2014;472(5):1409–15.
Juwara L, et al. Identifying predictive factors for neuropathic pain after breast cancer surgery using machine learning. Int J Med Inform. 2020;141: 104170.
Martinez V, et al. Risk factors predictive of chronic postsurgical neuropathic pain: the value of the iliac crest bone harvest model. Pain. 2012;153(7):1478–83.
Cutler RB, et al. Identifying patients at risk for loss to follow-up after pain center treatment. Pain Med. 2001;2(1):46–51.
Beyaz SG, et al. Chronic postsurgical pain and neuropathic symptoms after abdominal hysterectomy: a silent epidemic. Medicine (Baltimore). 2016;95(33): e4484.
Acknowledgements
We thank the participants of the study.
Funding
The present work was co-funded by the European Union and Greek national funds through the Operational Program “Human Resources Development, Education and Lifelong Learning” (NSRF 2014–2020), under the call “Supporting Researchers with an Emphasis on Young Researchers—Cycle B” in the context of the project “Effect of perioperative intravenous infusion of dexmedetomidine vs. lidocaine on postoperative pain and analgesic consumption, bowel function and recovery after abdominal gynecological surgery: a randomized double-blind study” (MIS: 5047961). No Rapid Service Fee was received by the journal for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Martina Rekatsina and Chryssoula Staikou contributed to the study conception and design. Material preparation, data collection and analysis were performed by Martina Rekatsina, Polyxeni Theodosopoulou, and Chryssoula Staikou. The first draft of the manuscript was written by Martina Rekatsina and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Polyxeni Theodosopoulou and Chryssoula Staikou have nothing to disclose. Martina Rekatsina is a member of the Journal Editorial Board.
Compliance with Ethics Guidelines
Ethical approval was provided by the Institutional Review Board (IRB) of Aretaieio University Hospital, Athens, Greece on 31st of January 2017. (Protocol ID: EE-2/04/31-01-2017, Chairman Dr I, Vassileiou). The trial was registered on the ClinicalTrials.gov (ID: NCT03363425). The study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. All subjects provided informed consent to participate in the study. All participants provided consent for publication if any identifying information is included in the manuscript.
Data Availability
All data generated during the current study are available on request by Martina Rekatsina at mrekatsina@gmail.com.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Rekatsina, M., Theodosopoulou, P. & Staikou, C. Perioperative Dexmedetomidine or Lidocaine Infusion for the Prevention of Chronic Postoperative and Neuropathic Pain After Gynecological Surgery: A Randomized, Placebo-Controlled, Double-Blind Study. Pain Ther 11, 529–543 (2022). https://doi.org/10.1007/s40122-022-00361-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00361-5