Abstract
Introduction
Haematological patients are at higher risk of bloodstream infections (BSI) after chemotherapy. The aim of this study was to develop a simulation model assessing the impact of selective digestive decontamination (SDD) of haematological patients colonised with extended-spectrum beta-lactamase-producing Enterobacterales (ESBL-E) on the incidence of ESBL-E BSI after chemotherapy.
Methods
A patient population was created by a stochastic simulation model mimicking the patients’ states of colonisation with ESBL-E during hospitalisation. A systematic literature search was performed to inform the model. All ESBL-E carriers were randomised (1:1) to either the intervention (targeted SDD) or the control group (placebo). ESBL-E BSI incidence was the outcome of the model. Sensitivity analyses were performed by prevalence of ESBL-E carriage at hospital admission (low: < 10%, medium: 10–25%, high: > 25%), duration of neutropenia after receiving chemotherapy, administration of antibiotic prophylaxis with quinolones, and time interval between SDD and chemotherapy.
Results
The model estimated that the administration of targeted SDD before chemotherapy reduces the incidence of ESBL-E BSI in the hospitalised haematological population up to 27%. The greatest benefit was estimated in high-prevalence settings, regardless of the duration of neutropenia, the time interval before chemotherapy, and the administration of antibiotic prophylaxis with quinolones (p < 0.05). In medium-prevalence settings, SDD was effective in patients receiving quinolone prophylaxis, with either 1-day time interval before chemotherapy and a neutropenia duration > 6 days (p < 0.05) or 7-day time interval before chemotherapy and a neutropenia duration > 9 days (p < 0.05). No benefit was observed in low-prevalence settings.
Conclusions
Our model suggests that targeted SDD could decrease the rate of ESBL-E BSI in haematological carriers before chemotherapy in the setting of high ESBL-E prevalence at hospital admission. These estimates require confirmation by well-designed multicentre RCTs, including the assessment of the impact on resistance/disruption patterns of gut microbiome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Haematological patients are at higher risk of bloodstream infections after undergoing chemotherapy. |
The aim of this study was to develop a simulation model assessing the impact of selective digestive decontamination (SDD) of haematological patients colonised with extended-spectrum beta-lactamase-producing Enterobacterales (ESBL-E) on the incidence of ESBL-E BSI after chemotherapy. |
The model estimations will drive future studies to assess the effect of targeted SDD on clinical, microbiological, and epidemiological outcomes, including the impact on resistance to antibiotics. |
What was learned from the study? |
The model estimated a reduction in the incidence of ESBL-E BSI after chemotherapy in the targeted SDD group: the greatest benefit was estimated in high-prevalence settings at hospital admission, regardless of the duration of neutropenia, the time interval before chemotherapy, and the administration of antibiotic prophylaxis with quinolones. |
Our model suggests that targeted SDD could decrease the rate of ESBL-E BSI in haematological carriers before chemotherapy in the setting of high ESBL-E prevalence at hospital admission. |
These estimates require confirmation by well-designed multicentre RCTs, including the assessment of the impact on resistance/disruption patterns of gut microbiome. |
Introduction
Neutropenia is the most frequent host-cell defect in patients affected by haematological malignancies and increases the risk of development of severe infections, such as bacterial and fungal sepsis [1, 2]. Recommendations from international guidelines suggest antibiotic prophylaxis in patients with high risk of prolonged neutropenia after chemotherapy [3, 4]. An updated systematic review with meta-analysis of fluoroquinolone-based antibacterial chemoprophylaxis in neutropenic patients with haematological malignancies from the European Conference on Infections in Leukaemia (ECIL) reported fewer febrile episodes (pooled odds ratio [OR] 0.32, 95% CI 0.20–0.50) and fewer bloodstream infections (OR 0.57, 95% CI 0.43–0.74) but no effect upon all-cause mortality (pooled OR 1.01, 95% CI 0.73–1.41) [5,6,7]. However, the effectiveness of prophylaxis is now under discussion because of the change in the epidemiological scenario of bloodstream infections (BSI) and the risk of promoting resistance among both gram-negative and gram-positive bacteria [8,9,10,11,12,13,14,15,16,17,18,19].
Due to an empty pipeline, infection control measures could play a pivotal role in controlling the spreading of ESBL-E in health-care settings [20]. Among them, decolonisation strategies, defined as any measure that leads to loss of ESBL-E detectable carriage and validated mainly in the intensive care unit (ICU) setting, seem promising [21,22,23,24,25,26]. The first assumption underlying decolonisation strategies is that colonisation increases the risk for subsequent infections [27].
To date, the role of SDD targeting ESBL-E has not been investigated in haematological neutropenic patients. We developed a stochastic simulation model to assess the impact of targeted SDD (decolonisation of ESBL-E carriers) on the incidence of ESBL-E BSI in patients with haematological malignancies after the first cycle of chemotherapy, according to the prevalence of ESBL-E carriage at hospital admission, the administration of antibiotic prophylaxis with quinolones, the time interval between SDD and chemotherapy, and the duration of neutropenia. The model estimations will drive future studies to assess the effect of targeted SDD on clinical, microbiological, and epidemiological outcomes, including the impact on resistance to antibiotics.
Methods
Baseline Model
We developed a stochastic simulation model which created a population of patients with haematological malignancies who were admitted to the haematological ward for the first cycle of chemotherapy, mimicking the patients’ states of colonisation and/or infection with ESBL-E during hospitalisation (Markov chain Monte Carlo simulation model) [28]. After admission, all ESBL-E carriers were randomised (1:1) to either the intervention (targeted SDD) or the control group (placebo). ESBL-E BSI incidence was the outcome in this model. To increase the internal validity, a second analysis of universal SDD (not targeting ESBL-E carriers) was performed (see Tables S1–S2–S3 in the electronic Supplementary Material).
Sensitivity analyses were performed to assess the impact of different parameters on the efficacy of SDD. The assessed parameters were the prevalence of ESBL-E carriage at hospital admission, the duration of neutropenia after receiving chemotherapy, the administration of antibiotic prophylaxis with quinolones, and the time interval between SDD and chemotherapy.
The model was developed and informed using both published evidence and unpublished data (local data recorded at the hematological department of the University of Groningen and unpublished data from the European-funded SATURN project [29]). A systematic literature search was performed to inform the model on the effect of decolonisation on carriers of ESBL-E. Articles were identified through computerised literature searches using PubMed, the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, and Web of Science and by reviewing the references of retrieved articles. Index search terms included: (Citrobacter OR Enterobacter OR Escherichia OR Klebsiella OR Morganella OR Proteus OR Providencia OR Serratia OR Enterobacteriaceae OR Enterobacterales OR coliform OR gram-negative bacteria) AND (cephalosporin-resistan* OR cephalosporin resistan* OR beta-lactamase OR beta lactamase) AND (decolon* OR decontamin* OR eradicat* OR suppress*). The search was restricted to full articles published in English up to 2019 including adult hospitalised patients (> 16 years of age). Articles reporting interventions in specific hospital settings not involving haematological patients (i.e. ICU, solid organ transplantation) were excluded.
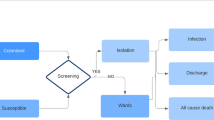
Patients could display five different states related to ESBL-E: susceptibility to colonisation (S); colonisation (C); ESBL-E BSI (I); decolonisation after SDD, ESBL-E BSI treatment, or spontaneously (D); death after ESBL-E BSI (X). Figure 1 shows the model pathways.
The model input parameters are reported in a dedicated section of the results and in Table 1.
As this article is based on previously conducted studies, it does not contain any new studies with human participants or animals performed by any of the authors.
Statistics and Modelling
A Markov chain Monte Carlo simulation was performed. The simulation model drew the number of patients in the ward, the absolute neutrophil count, and the patients' state related to ESBL-E. Daily probabilities of state transitions were assigned by taking the 30th fractional matrix root of the 30-day probabilities (or analogue the 7th/28th fractional matrix root of the 7/28 day-probabilities). Results were adapted using a stochastic matrix. Considering that the state transition model consisted of more than two states, a differential evolution algorithm was applied for optimisation [30]. State transition probabilities were adjusted dynamically, where necessary. The cohort models were applied to 75,000 patients per run (two runs: targeted SDD and universal SDD). Simulation data were summarized in pivot tables (see Table 2 and Table S1 in the electronic Supplementary Material). All the values of the variables were reported in a summary file per run. The overall results were combined automatically by the summary module. Two-sided p values < 0.05 were considered significant (Mann-Whitney-Wilcoxon test). p values were determined in cross-run comparisons to assess differences in ESBL-E BSI rates between different values of parameters (i.e. low vs. medium prevalence of ESBL-E carriage at hospital admission).
Results
Model Input Parameters
The systematic literature search identified one RCT [31] and one prospective cohort study [32] assessing the effectiveness of SDD on ESBL-E rectal carriage in adult hospitalised patients. Huttner et al. allocated 54 patients to either placebo or oral colistin sulphate (50 mg four times daily) and neomycin sulphate (250 mg four times daily) for 10 days (plus nitrofurantoin for 5 days in case of urine detection). A significantly lower rectal carriage was observed in the treatment group at the end of treatment, 32% (8/25) vs. 76.9% (20/26; p = 0.001). The effect turned out to be not significant at 7-day [66.7% (18/27) vs. 68% (17/25), p = 0.92] and at 28-day post-treatment [51.9% (14/27) vs. 37% (10/27), p = 0.28] [31]. In an 8-year prospective cohort study, Buehlmann et al. enrolled 35 asymptomatic ESBL-E carriers and treated with chlorhexidine mouth rinse for 4 days for throat colonisation, oral paromomycin for 4 days for rectal colonisation, or oral nitrofurantoin or fosfomycin (single dose) or ciprofloxacin or cotrimoxazole for 5 days for urinary colonisation. The course was repeated in patients with persistent ESBL-E carriage, showing that repeated decolonisation significantly improved eradication rate at treatment end [88.9% (16/18) vs. 41.1% (7/17); p = 0.007] [32]. No clinical outcomes were analysed in either of the two studies [31, 32]. On the basis of the higher level of evidence displayed by the RCT [31], the modelled SDD regimen consisted of oral colistin sulphate (50 mg four times daily) and neomycin sulphate (250 mg four times daily) for 10 days.
The modelled population consisted of patients with haematological malignancies who were admitted to a 37-bed haematological department at a tertiary care hospital for the first cycle of chemotherapy with a normal absolute neutrophil count and with a probability of being ESBL-E carriers given by the prevalence of ESBL-E at hospital admission (low: < 10%, medium: 10–25%, high: > 25%) [33]. The length of hospital stay was assumed to be 30 days [34, 35]. The SDD regimen, colistin sulphate (50 mg four times daily) and neomycin sulphate (250 mg four times daily) for 10 days, was administered at 1, 7, or 28 days before chemotherapy over 10 days [31]. After chemotherapy, patients were expected to develop neutropenia (< 500/µl), lasting 3, 6, 9, 12, or 15 days [3, 36]. It was simulated that 50% of the admitted population received fluoroquinolones as antibiotic prophylaxis [37].
Table 1 shows the estimated transition rates from one state to another during hospitalisation, based on data retrieved from the systematic literature search. During hospitalisation the daily rate of transition from one state to another was assumed constant [38]. The ESBL-E acquisition rate among susceptible patients was modelled taking into account patient-to-patient transmission (directly or through contaminated hands of healthcare workers) and environmental reservoirs [39]. The ESBL-E acquisition rate was assumed constant from environmental reservoirs and proportional to the colonisation rate in the ward considering patient-to-patient-transmission [38]. The model included a constant rate of spontaneous ESBL-E decolonisation.
Model Simulations
The effect of SDD on ESBL-E BSI incidence rate had the greatest benefit in the setting of high prevalence at hospital admission. In this setting, targeted SDD significantly reduced the ESBL-E BSI incidence rate, regardless of the duration of neutropenia (p < 0.05) (see Tables 2, 3, 4), the SDD time interval before chemotherapy (p < 0.03) (see Tables 2, 5, 6), and the administration of antibiotic prophylaxis (p < 0.05) (see Table 2 and Fig. 2a, b).
a ESBL-E BSI incidence, depending on the ESBL-E prevalence at hospital admission, in patients not receiving prophylaxis with quinolones. The grey plots show patients in the control arm. The white box plots show patients in the targeted SDD arm. b ESBL-E BSI incidence, depending on the ESBL-E prevalence at hospital admission, in patients receiving prophylaxis with quinolones. The grey plots show patients in the control arm. The white box plots show patients in the targeted SDD arm
In medium-prevalence settings, the difference in the ESBL-E BSI incidence rate between the targeted-SDD group and the control group turned out to be significant in patients receiving quinolone prophylaxis, with either 1-day SDD time interval before chemotherapy and a duration of neutropenia > 6 days (p < 0.05) or 7-day SDD time interval and a duration of neutropenia > 9 days (p < 0.05) (see Table 2). In low-prevalence settings, the difference between the two groups was not statistically significant, regardless of the time of SDD administration, administration of prophylaxis, or the duration of neutropenia (see Table 2).
Discussion
This simulation model in a haematological population suggests that the benefit of the targeted SDD administration before chemotherapy varies largely, according to the prevalence of ESBL-E carriage at hospital admission, the duration of neutropenia after receiving chemotherapy, the administration of antibiotic prophylaxis with quinolones, and the time interval between SDD and chemotherapy. The most significant effect was observed in high-prevalence settings, when SDD was provided 1 day before the beginning of chemotherapy in high-risk patients expecting prolonged neutropenia.
The results of this simulation model are consistent with the observation that ESBL-E colonisation increases the risk of ESBL-E BSI [13, 34]. A systematic review of the link between colonisation and subsequent ESBL-E infection in patients with solid or haematological malignancies, including 10 observational studies and 2211 patients, showed a 13-fold increase in infection risk associated with colonisation [13]. The rates of ESBL-E colonisation in haematological patients display a huge variability between different countries and geographic areas, ranging from 13% in Western Europe to 57% in Southeast Asia, and even between different hospitals within the same country or region [13]. A German prospective observational study in 497 haematological patients showed an ESBL-prevalence at hospital admission of 11% (mainly Escherichia coli), ranging from 6 to 23%, and identified previous colonisation with extended-spectrum β-lactamase-producing Enterobacterales (ESBL-E) as the most important risk factor for ESBL-E bloodstream infections (BSIs) [34].
Colonised patients enhance also the likelihood of horizontal transmission. Although the burden has not been specifically estimated in the haematological ward, active surveillance screening of multidrug-resistant strains and strict adherence to contact precautions in case of colonization or infection should be applied to prevent the further spread of these resistant pathogens in case of endemicity or outbreaks [36].
Longer duration of neutropenia has also been associated with an increased risk of BSI and other infections due to resistant pathogens [36, 40]. Current guidelines recommend antibiotic prophylaxis with quinolones in haematological high-risk patients with prolonged (> 7 days) or profound (absolute neutrophil count < 100/µl) neutropenia [3]. Literature displays limited evidence on the emergence of ESBL-producers associated with antibiotic prophylaxis with quinolones and retrieved results are contradictory. In institutions and geographic regions in which there are high rates of fluoroquinolone resistance, the use of these agents for prophylaxis is less likely to be effective [41, 42]. While most studies showed no significant impact on resistance rates, others reported an increase in infections caused by ESBL-E [5, 8,9,10,11]. In particular, quinolones have shown to reduce the rate of susceptible intestinal pathogens and promote the selection and growth of ESBL-E strains [43, 44]. In one prospective study, haematopoietic cell transplant recipients who were colonized with levofloxacin-resistant extended-spectrum beta-lactamase-producing Enterobacterales (ESBL-E) pre-transplant and received levofloxacin prophylaxis showed high rates of bacteraemia from their colonizing strains during neutropenia [45]. Interestingly, previous studies identified the prior use of quinolones as a significant risk factor associated with ESBL-E bacteraemia in cancer patients [46,47,48,49]. A recent multicentre prospective cohort study, assessing the impact of antibiotics on resistance selection at intestinal level, showed that the administration of monotherapy with quinolones ranked high in promoting ESBL-E colonisation [50]. This evidence is consistent with our results and could explain the greater benefit of targeted SDD on ESBL-E BSI incidence in both patients with prolonged neutropenia and haematological patients receiving antibiotic prophylaxis.
The most extensive experience in universal decolonisation (decolonisation of all patients without previous screening) is with oral non-absorbable selective digestive decontamination (SDD) in ICU patients. Randomised controlled trials (RCTs) performed in low and high endemicity settings of multidrug-resistant gram-negative bacteria showed that universal SDD was effective in reducing infections caused by multidrug-resistant gram-negative bacteria in both settings, with limited impact on selection for new resistance [22,23,24,25, 51, 52]. Only one randomized controlled trial assessed the effect of a targeted SDD regimen on ESBL-E carriage in a mixed hospital population, demonstrating a temporary reduction in the carriage rate [31]. Resistance induction or selection represents the major potential drawback of decolonisation. While some studies reported an increased development of resistance to decolonisation therapy, particularly pointing out the appearance of resistance in gram-negative bacteria after the administration of colistin [53,54,55,56], others showed no significant increase in antibiotic resistance [22, 51, 57,58,59,60,61]. In theory, SDD might increase the risk on selecting pan-resistant strains. On the other hand, high intraluminal levels of antibiotics exceed minimum inhibition concentrations of resistant pathogens, leading at least to temporary suppression, which reduces the risk of overgrowth and cross-transmission. Further studies with detailed microbiological surveillance are needed to determine the ecological safety of SDD.
This study is subject to limitations, mainly due to the limited available evidence on which model parameter values were based. A few, non-comparative studies, targeting carbapenem-resistant Enterobacterales only, evaluated the efficacy of SDD in the haematological setting [62,63,64,65,66]. The investigated SDD regimen relies on the only high-quality RCT assessing the effect of SDD on ESBL-E carriage, which was conducted in a mixed hospital population [31]. An ideal model would be constructed from a prospective registry of neutropenic patients treated with chemotherapy, in which data on a variety of clinical and epidemiological measures could be collected. The SDD duration was not investigated in this study, assuming a 10-day course of oral colistin and neomycin [31]. Nevertheless, based on the evidence for the temporary effectiveness of decolonisation on ESBL-E rectal carriage [31], it would be reasonable to start the SDD shortly before the neutropenia-inducing chemotherapy and to extend the administration until the resolution of neutropenia. Due to the lack of specific data, the model could not explore the SDD effect on either the selection of strains resistant to decolonising agents or microbiome disruption. For the same reason, only the first cycle of chemotherapy was considered, even though a possible subsequent development of resistance could minimise the SDD benefit in the following cycles.
Conclusions
Considering that rates of colonisation and infection with ESBL-E vary considerably between study sites, our data may support SDD in high-incidence settings, whereas the cost-benefit ratio may not be satisfactory in medium- or low-incidence settings. These estimates require confirmation by well-designed multicentre RCTs in the setting of high ESBL-E prevalence at hospital admission, administering targeted SDD shortly before the neutropenia-inducing chemotherapy. Future studies should be conducted to determine the effect of decolonisation strategies on clinical, microbiological, and epidemiological outcomes, including the assessment of the impact on resistance/disruption patterns of gut microbiome.
References
Schimpff SC. Empiric antibiotic therapy for granulocytopenic cancer patients. Am J Med. 1986;80:13–20.
Bodey GP, Buckley M, Sathe YS, et al. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64:328–40.
Taplitz RA, Kennedy EB, Bow EJ, et al. Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA Clinical Practice Guidline Update. J Clin Oncol. 2018;36:3043–54.
Neumann S, Krause SW, Maschmeyer G, et al. Primary prophylaxis of bacterial infections and Pneumocystis jirovecii pneumonia in patients with hematological malignancies and solid tumors: guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Ann Hematol. 2013;92:433–42.
Mikulska M, Averbuch D, Tissot F, et al. Fluoroquinolone prophylaxis in haematological cancer patients with neutropenia: ECIL critical appraisal of previous guidelines. J Infect. 2018;76:20–37.
Bucaneve G, Micozzi A, Menichetti F, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med. 2005;353:977–87.
Cullen M, Steven N, Billingham L, et al. Antibacterial prophylaxis after chemotherapy for solid tumors and lymphomas. N Engl J Med. 2005;353:988–98.
Craig M, Cumpston AD, Hobbs GR, et al. The clinical impact of antibacterial prophylaxis and cycling antibiotics for febrile neutropenia in a hematological malignancy and transplantation unit. Bone Marrow Transplant. 2007;39:477–82.
Cumpston A, Craig M, Hamadani M, et al. Extended follow-up of an antibiotic cycling program for the management of febrile neutropenia in a hematologic malignancy and hematopoietic cell transplantation unit. Transpl Infect Dis. 2013;15:142–9.
Macesic N, Morrissey CO, Cheng AC, et al. Changing microbial epidemiology in hematopoietic stem cell transplant recipients: increasing resistance over a 9-year period. Transpl Infect Dis. 2014;16:887–96.
Garnica M, Nouér SA, Pellegrino FLPC, et al. Ciprofloxacin prophylaxis in high risk neutropenic patients: effects on outcomes, antimicrobial therapy and resistance. BMC Infect Dis. 2013;13:356.
Kolar M, Htoutou Sedlakova M, Pudova V, et al. Incidence of fecal Enterobacteriaceae producing broad-spectrum beta-lactamases in patients with hematological malignancies. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:100–3.
Alevizakos M, Karanika S, Detsis M, et al. Colonisation with extended-spectrum β-lactamase-producing Enterobacteriaceae and risk for infection among patients with solid or haematological malignancy: a systematic review and meta-analysis. Int J Antimicrob Agents. 2016;48:647–54.
Young BE, Lye DC, Krishnan P, et al. A prospective observational study of the prevalence and risk factors for colonization by antibiotic resistant bacteria in patients at admission to hospital in Singapore. BMC Infect Dis. 2014;14:298.
Shitrit P, Reisfeld S, Paitan Y, et al. Extended-spectrum beta-lactamase-producing Enterobacteriaceae carriage upon hospital admission: prevalence and risk factors. J Hosp Infect. 2013;85:230–2.
Pasricha J, Koessler T, Harbarth S, et al. Carriage of extended-spectrum beta-lactamase-producing enterobacteriacae among internal medicine patients in Switzerland. Antimicrob Resist Infect Control. 2013;2:20.
Ruppé E, Pitsch A, Tubach F, et al. Clinical predictive values of extended-spectrum beta-lactamase carriage in patients admitted to medical wards. Eur J Microbiol Infect Dis. 2012;31:319–25.
Chabok A, Tärnberg M, Smedh K, et al. Prevalence of fecal carriage of antibiotic-resistant bacteria in patients with acute surgical abdominal infections. Scand J Gstroenterol. 2010;45:1203–10.
Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–27.
Tacconelli E, Cataldo MA, Dancer SJ, et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014;Suppl 1:1–55.
Daneman N, Sarwar S, Fowler RA, et al. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:328–41.
de Smet AMGA, Kluytmans JAJW, Blok HEM, et al. Selective digestive tract decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. Lancet Infect Dis. 2011;11:372–80.
Melsen WG, de Smet AMGA, Kluytmans JAJW, et al. Selective decontamination of the oral and digestive tract in surgical versus non-surgical patients in intensive care in a cluster-randomized trial. Br J Surg. 2012;99:232–7.
Sánchez-Ramírez C, Hípola-Escalada S, Cabrera-Santana M, et al. Long-term use of selective digestive decontamination in an ICU highly endemic for bacterial resistance. Crit Care. 2018;22:141.
Oostdijk EAN, Kesecioglu J, Schultz MJ, et al. Effects of decontamination of the oropharynx and intestinal tract on antibiotic resistance in ICUs. JAMA. 2014;312:1429–37.
Tacconelli E, Mazzaferri F, de Smet AM, et al. ESCMID-EUCIC clinical guidelines on decolonization of multidrug-resistant Gram-negative bacteria carriers. Clin Microbiol Infect. 2019;25:807–17.
Safdar N, Bradley EA. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med. 2008;121:310–5.
Hamra G, MacLehose R, Richardson D. Markov chain Monte Carlo: an introduction for epidemiologists. Int J Epidemiol. 2013;42:627–34.
De Angelis G, Restuccia G, Venturiello S, et al. Nosocomial acquisition of methicillin-resistant Staphyloccocus aureus (MRSA) and extended-spectrum beta-lactamase (ESBL) Enterobacteriaceae in hospitalised patients: a prospective multicenter study. BMC Infect Dis. 2012;12:74.
Chhatwal J, Jayasuriya S, Elbasha EH. Changing cycle lengths in state-transition models: doing it the right way. ISPOR Connect. 2014;20:12–4.
Huttner B, Haustein T, Uçkay I, et al. Decolonization of intestinal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae with oral colistin and neomycin: a randomized, double-blind, placebo-controlled trial. J Antimicrob Chemother. 2013;68:2375–82.
Buehlmann M, Bruderer T, Frei R, et al. Effectiveness of a new decolonisation regimen for eradication of extended-spectrum β-lactamase-producing Enterobacteriaceae. J Hosp Infect. 2011;77:113–7.
European Centre for Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe—annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2017. Stockholm: ECDC; 2018.
Vehreschild MJGT, Hamprecht A, Peterson L, et al. A multicentre cohort study on colonization and infection with ESBL-producing Enterobacteriaceae in high-risk patients with haematological malignancies. J Antimicrob Chemother. 2014;69:3387–92.
Arnan M, Gudiol C, Calatayud L, et al. Risk factors for, and clinical relevance of, faecal extended-spectrum β-lactamase producing Escherichia coli (ESBL-EC) carriage in neutropenic patients with haematological malignancies. Eur J Clin Microbiol Infect Dis. 2011;30:355–60.
Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:427–31.
Averbuch D, Tridello G, Hoek J, et al. Antimicrobial resistance in gram-negative rods causing bacteremia in hematopoietic stem cell transplant recipients: intercontinental prospective study of the infectious diseases working party of the European Bone Marrow Transplantation Group. Clin Infect Dis. 2017;65:1819–28.
Bar-Yoseph H, Hussein K, Braun E, et al. Natural history and decolonization strategies for ESBL/carbapenem-resistant Enterobacteriaceae carriage: systematic review and meta-analysis. J Antimicrob Chemother. 2016;71:2729–39.
Rodriguez-Bano J, Lopez-Cerero L, Navarro MD, et al. Faecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli: prevalence, risk factors and molecular epidemiology. J Antimicrob Chemother. 2008;62:1142–9.
Averbuch D, Orasch C, Cordonnier C, et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European conference on infections in leukaemia. Haematologica. 2013;98:1826.
Montassier E, Batard E, Gastinne T, et al. Recent changes in bacteremia in patients with cancer: a systematic review of epidemiology and antibiotic resistance. Eur J Clin Microbiol Infect Dis. 2013;32:841–50.
Cornejo-Juárez P, Pérez-Jiménez C, Silva-Sánchez J, et al. Molecular analysis and risk factors for Escherichia coli producing extended-spectrum β-lactamase bloodstream infection in haematological malignancies. PLoS ONE. 2012;7:e35780.
Paterson DL, Mulazimoglu L, Casellas JM, et al. Epidemiology of ciprofloxacin resistance and its relationship to extended-spectrum beta-lactamase production in Klebsiella pneumoniae isolates causing bacteremia. Clin Infect Dis. 2000;30:473–8.
Sohn KM, Kang C-I, Joo E-J, et al. Epidemiology of ciprofloxacin resistance and its relationship to extended-spectrum β-lactamase production in Proteus mirabilis bacteremia. Korean J Intern Med. 2011;26:89–93.
Satlin MJ, Chavda KD, Baker TM, et al. Colonization with levofloxacin-resistant extended-spectrum β-lactamase-producing Enterobacteriaceae and risk of bacteraemia in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2018;67:1720–8.
Ha YE, Kang C-I, Cha MK, et al. Epidemiology and clinical outcomes of bloodstream infections caused by extended-spectrum β-lactamase-producing Escherichia coli in patients with cancer. Int J Antimicrob Agents. 2013;42:403–9.
Rodríguez-Baño J, Navarro MD, Romero L, et al. Risk-factors for emerging bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Clin Microbiol Infect. 2008;14:180–3.
Oliveira AL, de Souza M, Carvalho-Dias VMH, et al. Epidemiology of bacteraemia and factors associated with multi-drug-resistant gram-negative bacteraemia in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2007;39:775–81.
Liss BJ, Vehreschild JJ, Cornely OA, et al. Intestinal colonisation and blood stream infections due to vancomycin-resistant enterococci (VRE) and extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBLE) in patients with haematological and oncological malignancies. Infection. 2012;40:613–9.
Tacconelli E, Gorska A, De Angelis G, et al. Estimating the association between antibiotic exposure and colonization with extended-spectrum beta-lactamase-producing Gram-negative bacteria using machine learning methods: a multicentre, prospective cohort study. Clin Microbiol Infect. 2020;26:87–94.
de Jonge E, Schultz MJ, Spanjaard L, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet. 2003;362:1011–6.
de Smet AMGA, Kluytmans JAJW, Cooper BS, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009;360:20–31.
Oostdijk EAN, Smits L, de Smet AMGA, et al. Colistin resistance in gram-negative bacteria during prophylactic topical colistin use in intensive care units. Intensive Care Med. 2013;39:653–60.
Halaby T, Al Naiemi N, Kluytmans J, et al. Emergence of colistin resistance in Enterobacteriaceae after the introduction of selective digestive tract decontamination in an intensive care unit. Antimicrob Agents Chemother. 2013;57:3224–9.
Lübbert C, Faucheux S, Becker-Rux D, et al. Rapid emergence of secondary resistance to gentamicin and colistin following selective digestive decontamination in patients with KPC-2-producing Klebsiella pneumoniae: a single-centre experience. Int J Antimicrob Agents. 2013;42:565–70.
Strenger V, Gschliesser T, Grisold A, et al. Orally administered colistin leads to colistin-resistant intestinal flora and fails to prevent faecal colonisation with extended-spectrum β-lactamase-producing enterobacteria in hospitalised newborns. Int J Antimicrob Agents. 2011;37:67–9.
Camus C, Salomon S, Bouchigny C, et al. Short-term decline in all-cause acquired infections with the routine use of a decontamination regimen combining topical polymyxin, tobramycin, and amphotericin B with mupirocin and chlorhexidine in the ICU: a single-center experience. Criti Care Med. 2014;42:1121–30.
Houben AJM, Oostdijk EAN, van der Voort PHJ, et al. Selective decontamination of the oropharynx and the digestive tract, and antimicrobial resistance: a 4-year ecological study in 38 intensive care units in the Netherlands. J Antimicrob Chemother. 2014;69:797–804.
Ochoa-Ardila ME, García-Cañas A, Gómez-Mediavilla K, et al. Long-term use of selective decontamination of the digestive tract does not increase antibiotic resistance: a 5-year prospective cohort study. Intensive Care Med. 2011;37:1458–65.
Noteboom Y, Ong DSY, Oostdijk EA, et al. Antibiotic-induced within-host resistance development of Gram-negative bacteria in patients receiving selective decontamination or standard care. Crit Care Med. 2015;43:2582–8.
van der Bij AK, Frentz D, Bonten MJM. Gram-positive cocci in Dutch ICUs with and without selective decontamination of the oropharyngeal and digestive tract: a retrospective database analysis. J Antimicrob Chemother. 2016;71:816–20.
Zuckerman T, Benyamini N, Sprecher H, et al. SCT in patients with carbapenem resistant Klebsiella pneumoniae: a single center experience with oral gentamicin for the eradication of carrier state. Bone Marrow Transplant. 2011;46:1226–30.
Lambelet P, Tascini C, Fortunato S, et al. Oral gentamicin therapy for Carbapenem-resistant Klebsiella pneumoniae gut colonization in hematologic patients: a single center experience. New Microbiol. 2017;40:161–4.
Kronman MP, Zerr DM, Qin X, et al. Intestinal decontamination of multidrug-resistant Klebsiella pneumoniae after recurrent infections in an immunocompromised host. Diagn Microbiol Infect Dis. 2014;80:87–9.
Brink AJ, Coetzee J, Corcoran C, et al. Emergence of OXA-48 and OXA-181 carbapenemases among Enterobacteriaceae in South Africa and evidence of in vivo selection of colistin resistance as a consequence of selective decontamination of the gastrointestinal tract. J Clin Microbiol. 2013;51:369–72.
Bilinski J, Grzesiowski P, Sorensen N, et al. Fecal microbiota transplantation in patients with blood disorders inhibits gut colonization with antibiotic-resistant bacteria: results of a prospective, single-center study. Clin Infect Dis. 2017;65:364–70.
Kang C-I, Chung DR, Ko KS, et al. Risk factors for infection and treatment outcome of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae bacteremia in patients with hematologic malignancy. Ann Hematol. 2012;91:115–21.
Reddy P, Malczynski M, Obias A, et al. Screening for extended-spectrum beta-lactamase-producing Enterobacteriaceae among high-risk patients and rates of subsequent bactereamia. Clin Infect Dis. 2007;45:846–52.
Fankhauser C, Zingg W, Francois P, et al. Surveillance of extended-spectrum-beta-lactamase-producing Enterobacteriaceae in a Swiss Tertiary Care Hospital. Swiss Med Wkly. 2009;139:747–51.
Tschudin-Sutter S, Frei R, Dangel M, et al. Rate of transmission of extended-spectrum beta-lactamase-producing Enterobacteriaceae without contact isolation. Clinical Infect Dis. 2012;55:1505–11.
Hilty M, Betsch BY, Bögli-Stuber K, et al. Transmission dynamics of extended-spectrum β-lactamase-producing Enterobacteriaceae in the tertiary care hospital and the household setting. Clin Infect Dis. 2012;55:967–75.
Acknowledgements
Funding
This study and the journal’s Rapid Service Fee was supported by clinical research funding of the Institute of Infectiology, Internal Medicine I, University Hospital Tübingen, Otfried-Müller-Straße 12, 72076 Tübingen, Germany (Institute VAT ID: DE 146 889 674). Funding was provided by Deutsches Zentrum für Infektionsforschung.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by all authors. The first draft of the manuscript was written by SD, FM, and ET. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Stefanie Döbele, Fulvia Mazzaferri, Tamara Dichter, Gerolf de Boer, Alex Friedrich, and Evelina Tacconelli have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Döbele, S., Mazzaferri, F., Dichter, T. et al. Modelling and Simulation of the Effect of Targeted Decolonisation on Incidence of Extended-Spectrum Beta-Lactamase-Producing Enterobacterales Bloodstream Infections in Haematological Patients. Infect Dis Ther 11, 129–143 (2022). https://doi.org/10.1007/s40121-021-00550-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-021-00550-3