Abstract
Purpose
Despite the evidence, the use of selective decontamination of the digestive tract (SDD) remains controversial, largely because of concerns that it may promote the emergence of antibiotic-resistant strains. The purpose of this study was to evaluate the long-term incidence of carriage of antibiotic-resistant bacteria (ARB), its clinical impact on developing infections and to explore risk factors of acquiring resistance.
Methods
This study was conducted in one 18-bed medical-surgical intensive care unit (ICU). All consecutive patients admitted to the ICU who were expected to require tracheal intubation for longer than 48 h were given a 4-day course of intravenous cefotaxime, and enteral polymyxin E, tobramycin, amphotericin B in an oropharyngeal paste and digestive solution. Oropharyngeal and rectal swabs were obtained on admission and once a week. Diagnostic samples were obtained on clinical indication.
Results
During 5 years 1,588 patients were included in the study. The incidence density of ARB was stable: 18.91 carriers per 1,000 patient-days. The incidence of resistant Enterobacteriaceae was stable; the resistance of Pseudomonas aeruginosa to tobramycin, amikacin and ciprofloxacin was strongly reduced; there was an increase of P. aeruginosa resistant to ceftazidime and imipenem, associated with the increase in imipenem consumption; the incidence of other nonfermenter bacilli and oxacillin-resistant Staphylococcus aureus was close to zero. Ninety-seven patients developed 101 infections caused by ARB: 23 pneumonias, 20 bloodstream infections and 58 urinary tract infections. Abdominal surgery was the only risk factor associated with ARB acquisition [risk ratio 1.56 (1.10–2.19)].
Conclusions
Long-term use of SDD is not associated with an increase in acquisition of resistant flora.
Similar content being viewed by others
Introduction
Selective decontamination of the digestive tract (SDD) is an antibiotic prophylaxis initially proposed to reduce the incidence of severe infections of lower airways [1, 2]. Further evidence demonstrated that SDD reduces bloodstream infections [3] and mortality [2–5] in critically ill patients requiring mechanical ventilation. From a strictly evidence-based perspective SDD should be recommended in mechanically ventilated patients because there is high-level clinical trial evidence supporting its use but only expert opinion against it [6]. Despite the evidence, the use of SDD remains controversial, largely because of concerns that it may promote the emergence of antibiotic-resistant strains. Two recent randomized controlled trials demonstrated that SDD does not increase resistance but actually reduces antibiotic resistance [4, 5]. Moreover five long-term SDD observational studies monitored antimicrobial resistance between 2 and 7 years, and reported that bacterial resistance associated with SDD was not a clinical problem [7–11]. These studies have some potential weaknesses: one study was a retrospective case–control study, two studies assessed the percentage of antibiotic-resistant isolates instead of the incidence of patients who acquired antibiotic-resistant bacteria (ARB) [9, 10], two studies specifically estimate the efficacy and safety of enteral vancomycin to eradicate oxacillin-resistant Staphylococcus aureus (ORSA) [7, 8]. The impact of potential confounding factors such as the prevalence of ARB on intensive care unit (ICU) admission, the frequency of sampling, and intrinsic factors associated with acquisition of resistance has been only evaluated in two trials [7, 8].
The main objective of this 5-year cohort study was to evaluate the incidence of ICU-acquired carriage of ARB and its time-trend in patients receiving SDD. Other secondary objectives were the clinical impact of resistance acquisition on developing infections due to ICU-acquired ARB and to explore some extrinsic and intrinsic risk factors for resistance acquisition.
Patients and methods
The study was a prospective cohort study conducted in an 18-bed medical-surgical ICU in a tertiary-care centre. The study population comprised all consecutive patients admitted to the ICU, who received SDD. SDD was administered to patients expected to require tracheal intubation for more than 48 h. The study period was 5 years (from March 2002 to February 2007).
All patients were included and all data were recorded on standardised documentation sheets. Admission data included demographics and SAPS II score [12].
All patients were treated four times daily with 1 g of an oral paste, applied to the oral cavity with a gloved finger by the patient’s nurse, containing 2% of polymyxin E, 2% of tobramycin, and 2% of amphotericin B. They also received 100 mg of polymyxin E, 80 mg of tobramycin, and 500 mg of amphotericin B into the gut administered through the nasogastric tube. In patients with a tracheostomy, the oral paste was also applied four times daily on the skin surrounding the tracheostomy. Enteral vancomycin, 4% oropharyngeal paste and 500 mg in digestive solution were added at the same 6-h interval to all ORSA carriers during the full ICU stay and to all patients coming from other hospitals or other wards of the hospital until they were deemed non-carriers of ORSA. For the first 4 days, systemic cefotaxime (1 g every 8 h) was administered to included patients [8, 13, 14]. SDD was started on the day of tracheal intubation and given throughout the treatment on the ICU.
Surveillance samples from throat, rectum, tracheostomy and pressure sores were obtained at the time of tracheal intubation, usually on admission, and once weekly thereafter. Diagnostic samples, such as tracheal aspirate, blood, urine, and wounds, were taken on clinical indication only. Standard methods for identification, typing, and susceptibility were used for all microorganisms [15]. Antimicrobial susceptibility testing was performed using the Wider system (Francisco Soria Melguizo, Spain, manufactured by Dade Behring Inc, West Sacramento, CA), a computer-assisted image-processing device adapted to read and interpret microdilution panels [16]. We adopted the antibiotic susceptibility and resistance breakpoints proposed by the MENSURA group [17]. The following criteria were used to define ARB: (a) Enterobacteriaceae resistant to cefotaxime and/or aminoglycosides and/or ciprofloxacin; (b) P. aeruginosa resistant to ceftazidime and/or aminoglycosides and/or ciprofloxacin and/or imipenem; (c) ORSA; (d) any strain of Acinetobacter sp. or other nonfermenters.
Primary endpoints were (a) the incidence of patients with samples—surveillance or diagnostic—positive for ARB acquired in the ICU; (b) the incidence of infections—lower airway, bloodstream and urinary tract—caused by ARB acquired in the ICU.
Copy strains defined as isolates with the same sensitivity pattern from the same site of the same patients at different times were excluded from the analysis.
Any ARB was considered to have been imported when surveillance or diagnostic samples were positive for it within 48 h of admission to the ICU. Acquisition was defined as isolation of a new strain not present in any of the samples taken in the first 2 days. A new case was defined as a patient known to be free of ARB, who subsequently had them isolated from a surveillance and/or diagnostic sample.
The diagnosis of pneumonia was based on clinical and microbiological criteria: a new or progressive infiltrate on chest radiographic examination, at least one of the following clinical symptoms: temperature at least 38.5°C, leukocytosis at least 12,000/μL, leucopenia less than 4,000/μL, and isolation of a potentially pathogenic microorganism in a semiquantitative culture of an endotracheal aspirate sample in concentrations of at least 105 colony forming units (CFU)/mL [18]. Other infections were diagnosed according to the Centers for Disease Control definitions in as far as they were applicable to ICU patients [19].
Infections were classified as secondary endogenous when they were preceded by gastrointestinal carriage of ARB with the same antibiogram and as exogenous when the infecting ARB was only isolated in the diagnostic sample without previous carriage [20].
Throughout the whole study period, high standards of infection control were maintained, in order to prevent transmission of resistant microorganisms via hands of healthcare workers. The infection control policy was based on (1) hand hygiene using 4% chlorhexidine; (2) isolation of the patients with ARB in a single-bedded side-room; (3) protective clothing; (4) care of equipment; and (5) cleanliness of the environment. Systemic antibiotics, cefotaxime plus an aminoglycoside, or imipenem, were administered empirically when clinical signs of infection developed and were adjusted according to the microbiology results. Infections owing to Gram-positive bacteria were treated with a β-lactam. Patients with infections caused by aerobic Gram-negative bacilli received a β-lactam or an aminoglycoside or carbapenem according to the microbiology results. Systemic vancomycin was given when the infection was caused by ORSA.
In a nested cohort of 431 consecutive patients severity score on admission, age, type of admission and co-morbidity were prospectively recorded to identify potential risk factors of ARB acquisition.
Antibiotic consumption was estimated by daily defined doses per 1,000 patient-days [21]. It represents the total ICU consumption of antibiotics of all patients admitted to the ICU—with and without SDD—during the study period.
Statistical analysis
The time-trend evolution of the incidence of acquired carriage was evaluated by Spearman regression coefficient [22]. Univariate analysis was used to compare demographics and risk factors between SDD-treated patients who did not acquire ARB and patients who acquired ARB.
This study met the exemption criteria of the ethics committee of clinical research because the data that were analysed were collected in the routine practice of the ICU and did not allow the identification of patients.
Results
During the 5 years 1,588 patients were included in the study, i.e. 34% of 4,681 patients who were admitted to the ICU in this period. The total length of stay of patients treated with SDD represents 75% of length of stay consumed by all the patients admitted to the ICU. The average length of stay of patients who received SDD was 12.45 days showing a significant decrease during the study period (r = −0.22; p < 0.001). Mean age was 63 years; 63% were male, mean SAPS II was 43, ICU mortality was 27%. All of these data were constant during the study period.
The frequency of sampling was 1,108 per 1,000 patient-days without significant changes upon time (p = 0.13). The consumption of parenteral antibiotics is shown in Table 1. There was a significant reduction in the consumption of ciprofloxacin and amikacin. The consumption of imipenem increased significantly.
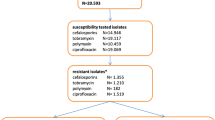
Among the patients who received SDD the prevalence of ARB on ICU admission was 22% and was stable during the study period. The cumulative incidence of ARB acquisition in this population was 23.6% with a significant time-trend reduction (r = −0.72; p = 0.01) (Fig. 1), but the incidence density (ID) was stable, 18.91 carriers per 1,000 patient-days. The cumulative incidence of antibiotic-resistant ICU-acquired Enterobacteriaceae was 9.5% [ID 7.6 per 1,000 patient-days; (r = 0.14; p = 0.71), P. aeruginosa 15.3% [ID 12.3 per 1,000 patient-days; (r = 0.34; p = 0.33)]. The distribution of imported and acquired ARB is shown in Table 2. In summary: (1) the incidence of Enterobacteriaceae resistant to the antimicrobials prescribed in SDD has been stable; (2) the resistance of P. aeruginosa to tobramycin, one component of SDD, and amikacin has been strongly reduced; (3) there has been a significant increase in the incidence of P. aeruginosa resistant to ceftazidime not related to the increase in prevalence on admission or to the increase of the consumption of cephalosporins; (4) the increase in the incidence of P. aeruginosa resistant to imipenem is time-associated with the increase in the consumption of imipenem (r = 0.71; p = 0.02); (5) the reduction of the incidence of P. aeruginosa resistant to ciprofloxacin is time-related with the reduction in the consumption of parenteral ciprofloxacin (r = 0.78; p = 0.01); (6) nonfermenter aerobic Gram-negative bacilli and ORSA show a close to null incidence.
Ninety-seven patients (6.1%) developed 101 infections caused by ICU-acquired ARB, which represents 5.1 infections per 1,000 patient-days. The distribution of infections and ARB is shown in Table 3. Twenty-three patients developed 23 episodes of pneumonia caused by ICU-acquired ARB: 4 episodes caused by Enterobacteraceae; 12 episodes caused by resistant P. aeruginosa; 1 caused by Acinetobacter sp.; 5 episodes caused by other nonfermenter bacilli; and 1 caused by ORSA. Twenty patients developed 20 bloodstream infections caused by ICU-acquired ARB: 5 episodes caused by resistant Enterobacteriaceae; 10 episodes caused by resistant P. aeruginosa; 1 caused by Acinetobacter sp.; 2 caused by other nonfermenters and 2 caused by ORSA. Fifty-four patients developed 58 urinary tract infections (UTI) caused by ICU-acquired ARB: 14 episodes were caused by Enterobacteraceae; 42 episodes by resistant P. aeruginosa; 1 by Acinetobacter sp. and 1 by other nonfermenters.
Thirteen pneumonias, 11 bloodstream infections and 41 UTI were classified as secondary endogenous (64%). Ten pneumonias, 9 bloodstream infections and 17 UTI were classified as exogenous (36%).
No cases of Clostridium difficile diarrhoea were diagnosed in patients receiving SDD.
The comparison between potential risk factors for acquiring resistance in the nested cohort of 431 consecutive patients is shown in Table 4. The only factor statistically associated with the acquisition of ARB in patients receiving SDD was abdominal surgery at or during ICU admission.
Discussion
Five main findings emerge from this 5-year study: (1) Long-term use of SDD is not associated with an increased resistance against the antimicrobials used as part of the SDD; in fact, a reduction in the incidence of P. aeruginosa resistant to aminoglycosides was observed. (2) Enterobacteriaceae did not show significant changes in the resistance pattern in 5 years, but ICU-acquired resistant P. aeruginosa showed an increase (imipenem) or reduction (ciprofloxacin) in resistance strongly associated with the parenteral consumption of these antibiotics. (3) The increase in carriers of P. aeruginosa resistant to ceftazidime was not associated with changes in ceftazidime consumption, nor with changes in prevalence at admission. (4) Long-term use of SDD resulted in a persistent rate close to 5 infections per 1,000 patient-days caused by ARB to commonly used antimicrobials and to antimicrobials used in SDD prophylaxis. (5) Two-thirds of infections were secondary endogenous, one-third exogenous. (6) Abdominal surgery is the only intrinsic factor found to be associated with acquisition of ARB.
Our findings are in line with randomized clinical trails [2, 5, 23] and with long-term observational studies [7–11] confirming that SDD does not increase resistance. Previous ecological studies have shown association between the consumption of systemic antibiotics and the resistance of Enterobacteriaceae and P. aeruginosa in the ICU [24]. In our study a time association has been found between antibiotic consumption in the ICU and reduction or increase in antibiotic resistance, but only limited to P. aeruginosa. The antibiotic resistance of Enterobacteriaceae did not change during the study period. The study design did not allow the identification of the potential causes of these findings.
The common denominator of the three potential sources of resistance is gut carriage of ARB. The enteral antimicrobials polymyxin, tobramycin and vancomycin effectively eradicated imported, and successfully prevented acquisition and de novo development of resistance [23, 25–28]. The proposed mechanism to explain the demonstrated efficacy of SDD to control the acquisition of antibiotic resistance is that faecal concentrations of enteral antimicrobials are usually high enough to exceed the minimal bactericidal concentration of ARB. In contrast, faecal concentrations of parenterally administered antimicrobials are often fluctuating following biliary excretion. Low antibiotic concentrations in the gut eradicate sensitive strains, selecting the resistant flora, increasing the risk of digestive colonization by ICU flora and the overgrowth of ARB [29]. Juan et al. [30] demonstrated that antibiotic resistance in P. aeruginosa isolates from ICU patients is mainly dependent on the selection of resistance mutations during therapy. By characterizing P. aeruginosa strains with molecular typing methods, the authors found that 21 patients with a previously susceptible strain harboured a strain resistant to the tested antibiotics, and in 20 of these 21 patients secondary resistance was a consequence of mutant selection since the susceptible and resistant isolates had the same DNA pattern.
An intriguing observation of the study is that in carriers of ARB, the global incidence of infections was kept at a low level, 5.1 infections per 1,000 patient-days. This finding is consistent with the results of de Smet et al. [31] who reported very low rates of bacteraemia (0.4%) and respiratory tract colonization (8%) by highly resistant pathogens in patients treated with SDD. Moreover they observed more than 50% reduction in bacteraemia caused by highly resistant pathogens in patients receiving SDD versus patients receiving standard care or oropharyngeal decontamination. These findings support the hypothesis that the risk of developing severe infections caused by ARB is low in patients treated with SDD. It has been postulated that the risk of infections caused by ARB is not only related to previous carriage but also to their overgrowth concentrations in the digestive tract. A reduction in carriage of ARB in overgrowth concentrations, as is found in SDD-treated patients [8], consequently reduces the incidence of infection caused by ARB.
This study showed that abdominal surgery was the only significant risk factor for acquisition of ARB. There are a number of studies supporting the association of colonization by P. aeruginosa with abdominal surgery or altered intestinal motility. In the study by Obritsch et al. [32], the percentage of patients infected or colonized by P. aeruginosa was higher amongst those with abdominal surgery (7.9%), when compared with thoracic-vascular surgery patients (4.8%) or trauma patients (4%). Ohara et al. [33] reported that among 32 patients with gastrointestinal colonization due to P. aeruginosa, 11 (34%) were diagnosed with ileus and 3 (9%) underwent gastrostomy when colonized, suggesting that abnormal gastrointestinal motility may be a risk factor for gastrointestinal colonization due to P. aeruginosa. Ileus and interrupted anatomy of the digestive tract explain the failure of SDD to prevent gut carriage of ARB because enteral antibiotics do not achieve bactericidal concentration in the lower part of the gut. Secondary endogenous infections—64% of infections caused by ARB acquired in the ICU—must be considered a limitation of SDD efficacy in some patients with impaired digestive tract function. Exogenous infections (36%) should be interpreted as a consequence of hygiene breaches [14]. Pneumonias and bloodstream infections show a similar proportion of secondary endogenous and exogenous infections, but secondary endogenous UTI are double the exogenous, reflecting that rectal flora, usually associated with UTI, is more difficult to eradicate than oropharyngeal flora, which, in general, causes pneumonia.
Interestingly, de Smet et al. [5] in their recent multicentre trial added polymyxin–tobramycin–amphotericin suppositories to achieve successful rectal clearance in patients with impaired gut anatomy and motility. In that Dutch trial resistance was successfully prevented using SDD, underlining the importance of regular surveillance of faecal flora to assess the efficacy of SDD and the need for additional interventions such as suppositories in patients with blind bowel loops or laxatives in patients with constipation [34] to achieve rectal clearance of potential pathogens. Although not proven, these measures may have added to the beneficial effects of SDD [4].
The strengths of this study is the large sample, 1,588 patients, 19,755 patient-days, the long-term use of SDD, the control of potential confounding factors related to the ICU such as the prevalence of carriers of resistant flora at ICU admission, the frequency of sampling and the consumption of parenteral antibiotics, and the assessment of potential individual risk factors in a large sample of 431 patients.
The main limitation of our study is the lack of molecular biotyping methods to determine the identity of the isolates, thus neither cross-contamination from other colonized patients, nor mutation or identification of an environmental source could be detected. Another potential weakness is the absence of a control group, but the aim of the study was not to compare the incidence of acquiring resistance in patients with or without SDD. It has been consistently demonstrated in randomized clinical trials that SDD significantly reduces resistance. The objective of the study was to assess the long-term effect on resistance acquisition in patients receiving SDD.
In conclusion this observational study supports the safety of SDD. The increase in ceftazidime-resistant P. aeruginosa deserves further evaluation in appropriately designed long-term SDD studies.
References
Baxby D, van Saene HK, Stoutenbeek CP, Zandstra DF (1996) Selective decontamination of the digestive tract: 13 years on, what it is and what it is not. Intensive Care Med 22:699–706
Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E (2009) Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev. doi:10.1002/14651858.CD000022.pub3
Silvestri L, van Saene HK, Milanese M, Gregori D, Gullo A (2007) Selective decontamination of the digestive tract reduces bacterial bloodstream infection and mortality in critically ill patients. Systematic review of randomized, controlled trials. J Hosp Infect 65:187–203
de Jonge E, Schultz MJ, Spanjaard L, Bossuyt PM, Vroom MB, Dankert J, Kesecioglu J (2003) Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet 362:1011–1016
de Smet AM, Kluytmans JA, Cooper BS, Mascini EM, Benus RF, van der Werf TS, van der Hoeven JG, Pickkers P, Bogaers-Hofman D, van der Meer NJ, Bernards AT, Kuijper EJ, Joore JC, Leverstein-van Hall MA, Bindels AJ, Jansz AR, Wesselink RM, de Jongh BM, Dennesen PJ, van Asselt GJ, te Velde LF, Frenay IH, Kaasjager K, Bosch FH, van Iterson M, Thijsen SF, Kluge GH, Pauw W, de Vries JW, Kaan JA, Arends JP, Aarts LP, Sturm PD, Harinck HI, Voss A, Uijtendaal EV, Blok HE, Thieme Groen ES, Pouw ME, Kalkman CJ, Bonten MJ (2009) Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med 360:20–31
Laupland KB, Fisman DN (2009) Selective digestive tract decontamination: a tough pill to swallow. Can J Infect Dis Med Microbiol 20:9–11
Cerda E, Abella A, de la Cal MA, Lorente JA, Garcia-Hierro P, van Saene HK, Alia I, Aranguren A (2007) Enteral vancomycin controls methicillin-resistant Staphylococcus aureus endemicity in an intensive care burn unit: a 9-year prospective study. Ann Surg 245:397–407
de la Cal MA, Cerda E, van Saene HK, Garcia-Hierro P, Negro E, Parra ML, Arias S, Ballesteros D (2004) Effectiveness and safety of enteral vancomycin to control endemicity of methicillin-resistant Staphylococcus aureus in a medical/surgical intensive care unit. J Hosp Infect 56:175–183
Heininger A, Meyer E, Schwab F, Marschal M, Unertl K, Krueger WA (2006) Effects of long-term routine use of selective digestive decontamination on antimicrobial resistance. Intensive Care Med 32:1569–1576
Leone M, Albanese J, Antonini F, Nguyen-Michel A, Martin C (2003) Long-term (6-year) effect of selective digestive decontamination on antimicrobial resistance in intensive care, multiple-trauma patients. Crit Care Med 31:2090–2095
Sarginson RE, Taylor N, Reilly N, Baines PB, van Saene HK (2004) Infection in prolonged pediatric critical illness: a prospective four-year study based on knowledge of the carrier state. Crit Care Med 32:839–847
Le Gall Jr, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
de la Cal MA, Cerda E, Hierro Garcia, van Saene HK, Gomez-Santos D, Negro E, Lorente JA (2005) Survival benefit in critically ill burned patients receiving selective decontamination of the digestive tract: a randomized, placebo-controlled, double-blind trial. Ann Surg 241:424–430
Taylor N, van Saene HK, Abella A, Silvestri L, Vucic M, Peric M (2007) Selective digestive decontamination. Why don’t we apply the evidence in the clinical practice? Med Intensiva 31:136–145
National Committee for Clinical Laboratory Standards (1990) Performance standards for antimicrobial susceptibility testing: NCCLS approved standard M100-S9. Wayne
Canton R, Perez-Vazquez M, Oliver A, Sanchez del Saz B, Gutierrez MO, Martinez-Ferrer M, Baquero F (2000) Evaluation of the Wider system, a new computer-assisted image-processing device for bacterial identification and susceptibility testing. J Clin Microbiol 38:1339–1346
Grupo MENSURA (2000) Recommendations from MENSURA for selection of antimicrobial agents for susceptibility testing and criteria for the interpretation of antibiograms. Rev Esp Quimioter 13:73–86
Ruiz M, Torres A, Ewig S, Marcos MA, Alcon A, Lledo R, Asenjo MA, Maldonaldo A (2000) Noninvasive versus invasive microbial investigation in ventilator-associated pneumonia: evaluation of outcome. Am J Respir Crit Care Med 162:119–125
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM (1988) CDC definitions for nosocomial infections, 1988. Am J Infect Control 16:128–140
de la Cal MA, Cerda E, Garcia-Hierro P, Lorente L, Sanchez-Concheiro M, Diaz C, van Saene HK (2001) Pneumonia in patients with severe burns: a classification according to the concept of the carrier state. Chest 119:1160–1165
Intensive Care Antimicrobial Resistance Epidemiology (ICARE) Surveillance Report, data summary from January 1996 through December 1997 (1999) A report from the National Nosocomial Infections Surveillance (NNIS) System. Am J Infect Control 27:279–284
Rumeau-Rouquette C, Breart G, Padieu R (eds) (1994) Répartition chronologique d’une variable. In: Méthodes en épidémiologie. Flammarion Médicine-Sciences, Paris, pp 268–283
Brun-Buisson C, Legrand P, Rauss A, Richard C, Montravers F, Besbes M, Meakins JL, Soussy CJ, Lemaire F (1989) Intestinal decontamination for control of nosocomial multiresistant gram-negative bacilli. Study of an outbreak in an intensive care unit. Ann Intern Med 110:873–881
Neuhauser MM, Weinstein RA, Rydman R, Danziger LH, Karam G, Quinn JP (2003) Antibiotic resistance among gram-negative bacilli in US intensive care units: implications for fluoroquinolone use. JAMA 289:885–888
Agusti C, Pujol M, Argerich MJ, Ayats J, Badia M, Dominguez MA, Corbella X, Ariza J (2002) Short-term effect of the application of selective decontamination of the digestive tract on different body site reservoir ICU patients colonized by multi-resistant Acinetobacter baumannii. J Antimicrob Chemother 49:205–208
Corbella X, Pujol M, Ayats J, Sendra M, Dominguez MA, Ardanuy C, Linares J, Ariza J, Gudiol F (1996) Relevance of digestive tract colonization in the epidemiology of nosocomial infections due to multiresistant Acinetobacter baumannii. Clin Infect Dis 23:329–334
Silvestri L, Milanese M, Oblach L, Fontana F, Gregori D, Guerra R, van Saene HK (2002) Enteral vancomycin to control methicillin-resistant Staphylococcus aureus outbreak in mechanically ventilated patients. Am J Infect Control 30:391–399
Viviani M, van Saene HK, Dezzoni R, Silvestri L, Di Lenarda R, Gullo G, Berlot A (2005) Control of imported and acquired methicillin-resistant Staphylococcus aureus (MRSA) in mechanically ventilated patients: a dose-response study of enteral vancomycin to reduce absolute carriage and infection. Anaesth Intensive Care 33:361–372
van Saene HK, Taylor N, Damjanovic V, Sarginson RE (2008) Microbial gut overgrowth guarantees increased spontaneous mutation leading to polyclonality and antibiotic resistance in the critically ill. Curr Drug Targets 9:419–421
Juan C, Gutierrez O, Oliver A, Ayestaran JI, Borrell N, Perez JL (2005) Contribution of clonal dissemination and selection of mutants during therapy to Pseudomonas aeruginosa antimicrobial resistance in an intensive care unit setting. Clin Microbiol Infect 11:887–892
de Smet AM, Kluytmans JA, Blok HE, Mascini EM, Benus RF, Bernards AT, Kuijper EJ, Leverstein-van Hall MA, Jansz AR, de Jongh BM, van Asselt GJ, Frenay IH, Thijsen SF, Conijn SN, Kaan JA, Arends JP, PDj Sturm, Bootsma MC, Bonten MJ (2011) Selective digestive tract decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. Lancet Infect Dis 11:372–380
Obritsch MD, Fish DN, MacLaren R, Jung R (2004) National surveillance of antimicrobial resistance in Pseudomonas aeruginosa isolates obtained from intensive care unit patients from 1993 to 2002. Antimicrob Agents Chemother 48:4606–4610
Ohara T, Itoh K (2003) Significance of Pseudomonas aeruginosa colonization of the gastrointestinal tract. Intern Med 42:1072–1076
van der Spoel JI, Oudemans-van Straaten HM, Kuiper MA, van Roon EN, Zandstra DF, van der Voort PH (2007) Laxation of critically ill patients with lactulose or polyethylene glycol: a two-center randomized, double-blind, placebo-controlled trial. Crit Care Med 35:2726–2731
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ochoa-Ardila, M.E., García-Cañas, A., Gómez-Mediavilla, K. et al. Long-term use of selective decontamination of the digestive tract does not increase antibiotic resistance: a 5-year prospective cohort study. Intensive Care Med 37, 1458–1465 (2011). https://doi.org/10.1007/s00134-011-2307-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2307-0