Abstract
Introduction
Generalized myasthenia gravis (gMG) is a rare autoimmune disease. Symptoms of gMG are diverse, and understanding of their impact on patients is limited. This qualitative study aimed to provide an in-depth exploration of patients’ daily experiences of gMG.
Methods
Published qualitative studies were reviewed to identify the most important signs, symptoms, and functional impacts related to the patient experience in gMG. Semi-structured hybrid concept elicitation interviews (allowing spontaneous generation of disease concepts) and cognitive debriefing interviews (assessing the validity of existing disease assessments) were conducted with clinicians and adult patients with gMG. Signs, symptoms, and impacts were reviewed to understand which were most salient (i.e., reported by at least 50% of patients, with disturbance rating 5 or higher [10-point numeric scale]); concept saturation was also assessed. The disease conceptual model was updated. Existing clinical outcomes assessments (COAs) that capture how patients feel, function, and survive were assessed.
Results
Interviews with patients (n = 24) identified seven new signs and symptoms and 37 new impacts compared with the literature. Concept saturation was reached. Signs and symptoms identified by patients as most important (salient) were shortness of breath, general fatigue, muscle weakness of arms, legs, and neck, dysphonia, dysarthria, trouble swallowing liquids, choking, and heat sensitivity. Patient-identified salient impacts were work life, depression, difficulty walking, grooming hair, showering, and brushing teeth, eating, personal relationships, family life, and participating in social activities. Clinicians considered ocular, respiratory, swallowing, speech/talking, and extremity function as key clinical manifestations of gMG. Patients and clinicians found clinical outcome assessments (COAs) to be conceptually relevant and comprehensive.
Conclusion
This research provides a holistic understanding of gMG signs, symptoms, and impacts experienced by patients, as observed by patients and clinicians. The conceptual model of gMG highlights the range of signs, symptoms, and impacts that adult patients with gMG experience in their everyday lives, emphasizing the humanistic impact and unmet needs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Generalized myasthenia gravis (gMG) is a rare autoimmune disease of the neuromuscular junction affecting approximately 200 per 1 million people. It is characterized by muscle weakness and fatiguability that varies in severity and affected muscle group. Symptoms are progressive throughout the day, requiring hospitalization in severe cases. |
This research aimed to (1) characterize the most relevant signs, symptoms, and impacts of the patient experience with gMG; (2) establish the conceptual model of the patient experience with gMG, and (3) explore the content validity for existing clinical outcome assessments. |
This research emphasizes the voice of the patient, highlighting the everyday experience of adult patients with gMG; seven new signs and symptoms and 37 new impacts were identified, and the most salient signs, symptoms, and impacts were identified. |
The disease conceptual model was updated to give a holistic, visual representation of gMG. Clinical outcome assessments were found to be clinically relevant, with no missing elements. |
These findings may aid in informing a patient-centric and holistic measurement strategy within future research, clinical practice, and/or patient interventions. |
Introduction
Generalized myasthenia gravis (gMG) is a rare, chronic, autoimmune disease of the neuromuscular junction, affecting 14–20 out of every 100,000 people in the USA, with a worldwide prevalence of approximately 200 per 1 million people [1]. Clinically, gMG is characterized by fluctuating muscle weakness, fatiguability with mild physical activity/as the day progresses, and in severe cases hospitalization [1]. Symptoms are due primarily to the production of antibodies that bind the acetylcholine receptor, resulting in complement-mediated damage to the neuromuscular junction [2, 3].
In gMG, muscle weakness affects ocular, bulbar, and extremity function to various degrees, which in turn impairs many aspects of patients’ daily lives [4,5,6,7,8,9]. While the focus for healthcare professionals is currently clinically focused, understanding the patient experience of gMG is important to fully support patients living with the disease. Although current literature describing the signs, symptoms, and impacts experienced by adult patients with gMG is growing, understanding of their relevance, particularly in relation to patients’ everyday life, is still limited [5,6,7,8, 10,11,12].
The recent 21st Century’s Cures Act and the release of several patient-focused drug development (PFDD) guidance documents emphasize the importance of the patient perspective [13,14,15]. The overall aim is to ensure a patient-focused approach to drug development and decision-making; development of a treatment should meaningfully address the aspects of disease that are most important to patients, and studies should be tailored to patients’ needs and priorities. Combined with similar guidance from other regulators, health technology assessments, the clinical and scientific community and patient advocates, it has been established that the patient voice is paramount, particularly for diseases such as gMG where published data are limited [16,17,18,19].
With a heightened awareness of the importance of understanding how patients feel, function, and survive, this paper explores the results of an in-depth qualitative study exploring patients’ daily experience of gMG. In parallel, a review of the clinical outcome assessments (COAs) used in gMG was performed, including a critical appraisal of the measurement properties of three COAs in line with US Food and Drug Administration (FDA) guidance recommendations for COAs to support labeling claims [20, 21]: Myasthenia Gravis-Activities of Daily Living (MG-ADL) [22]; Quantitative Myasthenia Gravis (QMG) [23]; and Myasthenia Gravis Composite (MGC) [24] (Supplementary Material, Table S1).
This research aimed to (1) characterize the most relevant signs, symptoms, and impacts of the patient experience with gMG; (2) establish the conceptual model of the patient experience with gMG, and (3) explore the content validity for the three COAs outlined above to ensure they comprehensively cover all concepts most relevant to patients with gMG and to ensure that no fundamental elements are missing.
Methods
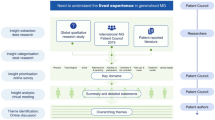
This qualitative study was conducted in two phases to develop a conceptual model of gMG. In phase 1, a comprehensive literature search was conducted from which a semi-structured discussion guide to investigate the signs, symptoms, and impact in patients with gMG was developed. In phase 2, semi-structured hybrid concept elicitation (CE) and cognitive debriefing (CD) interviews were conducted with clinicians and adult patients with gMG to generate an in-depth understanding of their experience of the disease. CE interviews allow spontaneous concepts to emerge by asking open-ended questions in a non-leading manner, whereas CD interviews assess the validity of existing assessments with the intention of understanding if every aspect of the instrument is relevant and meaningful to patients and nothing fundamental is missing [25, 26]. The signs, symptoms, and impacts identified were reviewed to understand which were most salient. Based on findings from both phases, a final conceptual model of disease was developed.
Phase 1: Literature Review
Initial research included a targeted literature review of published qualitative studies in gMG to identify signs, symptoms, and functional impacts of gMG (search strategy contained within Supplementary Material Table S2). A search of the PubMed biomedical database was performed in September 2020 using the following search terms: myasthenia gravis AND (qualitative research, interviews, narratives, focus groups, patient experience, grounded or phenomenological research, endpoint model, concepts, patient report, or self-report), limited to articles in English published in the last 10 years, and with an abstract available.
Peer-reviewed qualitative research studies focusing on adult (at least 18 years of age) patient experience of gMG were included. Articles were excluded if they focused primarily on experience of treatment, pathogenesis, genetics, or molecular biology of MG (full list of criteria in Supplementary Material Table S2). Abstracts that met all the inclusion and none of the exclusion criteria were included for closer review of the full article. Data focused on signs, symptoms, and impacts of gMG from each article meeting the eligibility criteria was extracted into Excel and used to inform a semi-structured discussion guide for use in qualitative interviews with clinicians and patients.
Phase 2: Qualitative Interviews
Qualitative Evidence Generation
Following the initial literature review, four streams of qualitative research were conducted using an iterative, data-driven approach to provide a comprehensive understanding of the patient and clinician experience of gMG. The iterative interview process was conducted in the following order: (1) clinician CE and CD interviews (with a focus on CE); (2) patient CE and CD interviews (with a focus on CE); (3) clinician CD interviews; (4) patient CE and CD (with a focus on CD). Interviews were conducted by two experienced interviewers from IQVIA: a doctoral-level medical anthropologist with at least 20 years of interview experience and a health-related market researcher with at least 20 years of experience conducting interviews. All interviews were conducted via telephone and via a web-based platform (Mercuri) to project the COAs. No patients or clinicians participated in both rounds of interviews. Semi-structured interview guides were developed on the basis of the literature review and followed an iterative process for both the clinician and the patient interviews to allow for refinement and refocusing of the research objectives, as needed.
Clinician Interviews
All first-round clinicians were recruited by an external partner Atheneum who retains a panel of clinicians who opt-in to participate in research studies. Licensed, currently practicing neurologists and specialists with 5–30 years of clinical experience, treating at least two patients with gMG monthly were targeted for interviews using a screener. The sample size was deemed sufficient to achieve concept saturation. Hybrid CE interviews (to allow spontaneous generation of disease concepts) and CD interviews (to challenge the validity of existing disease assessments) were conducted in two rounds. An interview guide was developed to guide each clinician interview specific to objectives to further characterize the patient experience of gMG and debrief legacy MG instruments with clinicians to gather any feedback based on their experience seeing and treating patients with gMG. The first round was conducted between July and August 2021 and the second between March and April 2022.
The first round aimed to elicit insights into the experience and treatment of patients with gMG in clinical practice. The second round involved clinicians who completed didactic training on administration of the three COAs (MG-ADL, MGC, and QMG), to understand the clinicians’ perceptions regarding the ease of administration, comprehensibility, and relevance. Round 1 and 2 interviews lasted approximately 90 and 60 min, respectively.
Patient Interviews
Qualitative interviews were designed to reach concept saturation and were completed with a total of 24 patients. Patients were identified and recruited from Rare Patient Voice and The Mast Cell Disease Society. Inclusion criteria were designed to represent the broader population of patients with gMG: at least 18 years of age, confirmed diagnosis of gMG, Myasthenia Gravis Foundation of America Clinical Classification Class II to IVa [27], not experiencing muscle weakness due to conditions other than gMG, willing to participate in a 90-min telephone interview, and fluent in English. The two partner recruitment agencies screened patients according to a study-specific screener. Hybrid CE/CD interviews were conducted in two rounds: the first between July and September 2021 and the second between February and March 2022. Patient interviews were conducted using a semi-structured guide beginning with open-ended questions to allow patients to spontaneously mention their signs, symptoms, and impacts of gMG, and probes were used when participants did not spontaneously mention of any concepts uncovered from the literature review and/or clinician interviews. Each interview lasted approximately 90 min. Interviewers used reflexivity to limit biasing interpretation of feedback based on their own beliefs, judgments, and practices.
The semi-structured discussion guides were designed specific to each round of interviews. In the first round, objectives were to (1) understand patient experiences with the disease and its impact on their daily lives, including signs, symptoms, and impacts perceived as most bothersome and disturbing (rated by patients from 0 to 10, where 0 = not bothersome/disturbing at all and 10 = extremely bothersome/disturbing), and (2) determine whether the concepts in the three COAs were relevant to their experience. The second round of interviews was designed to confirm findings from the first round, and allow for more comprehensive CD of the COAs, specifically, to ensure that the concepts and recall periods were relevant, comprehensive, and easy to understand to patients with gMG.
Data Analysis
Clinician Interviews
Clinician transcripts were qualitatively analyzed using Microsoft® Excel® (Redmond, WA) to expedite the data analysis process. Feedback was categorized into themes, including establishment of gMG diagnosis in routine practice, description of a typical patient with gMG seen in practice, and follow-up and assessment of disease progression/disease control over time.
Patient Interviews
Interviews were audio-recorded with each patient’s permission. Data from patient transcripts, including ratings of symptom bothersomeness or impact disturbance, were summarized using descriptive statistics.
De-identified verbatim transcripts were analyzed using inductive coding techniques, where codes are developed from qualitative data and amended as themes emerge, and deductive coding techniques, where pre-existing codes are used to analyze data on the basis of established theories, as well as content and thematic analysis to identify patterns in the data using software specifically designed for qualitative analysis, MAXQDA Plus, V. 20.3.0 (VERBI Software, 2020) [28, 29]. Two researchers trained in qualitative research methods were involved in the coding process, overseen by a senior doctoral-level researcher with at least 20 years’ experience in qualitative research. The team collaborated to reach consensus on any changes made to the coding frame. Dual coding took place on a minimum of 25% of transcripts to ensure consistency across coders.
Concept Saturation
Concept saturation is defined as the point at which no new concepts are introduced during interviews [13]. Interviews were organized chronologically and reviewed in three groups of four patients each to determine saturation for signs, symptoms, and impacts. Concepts mentioned by each group of patients were compared with the concepts mentioned in the previous group of interviews; if new concepts appeared, saturation was considered not achieved. The assessment of saturation was performed in the first round of interviews only.
Salience Analysis
Saliency of symptom and impact concepts were assessed and defined as those reported by at least 50% of patients, with a disturbance rating of 5 or higher on a numeric rating scale (with 0 being not severe at all and 10 being extremely severe). This definition was determined by the IQVIA study team, as it captures the top 25% most impactful concepts. To ensure no key concepts were left out of the saliency analysis, the study team reviewed the concepts identified by this numerical method and discussed whether any other critical concepts mentioned during the interviews should also be deemed salient. This discussion was informed by the team’s experience with gMG, patient interviews, and the literature review.
Conceptual Model of gMG
Data arising from interviews were contextualized with evidence from the prior qualitative research findings of the literature review. Findings from all interviews were used to update and finalize the conceptual disease model of the patient experience of living with gMG [25, 28]. This model is a visual representation of the daily experience of gMG, grouped by signs and symptoms, immediate impacts, and general impacts, which allows for a comprehensive understanding of the patient experience from both clinicians’ and patients’ perspectives to emerge.
Ethics Approval
The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and are consistent with Good Clinical Practice and applicable regulatory requirements. The study was reviewed and approved by the WCG institutional review board (IRB), IRB number 20213567. The research protocol, interview guides, all documents associated with patient interviews, and all clinician communication documents were also reviewed and approved by WCG IRB. Informed, written consent was obtained electronically from each participant prior to their enrollment.
This study was completed to a level of rigor in line with FDA patient-reported outcomes guidance documents, such as the 21st Century Cures Act and PFDD guidance, which places emphasis on the patient perspective [13,14,15].
Data Availability
Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the product and indication has been approved by major health authorities (e.g., FDA, European Medicines Agency, Pharmaceuticals and Medical Devices Agency), if there is legal authority to share the data and there is not a reasonable likelihood of participant re-identification. Submit requests to https://vivli.org/.
Results
Phase 1: Literature Review
Details of the article selection process for the targeted literature review are given in Supplementary Material Fig. S1 and Table S3. The findings revealed that published qualitative studies describing patients’ real-world experiences with gMG were sparse and did not identify the most relevant signs, symptoms, and impacts, although the authors note that there have been additional publications since the review was completed [12, 30]. A discussion guide for interviews was then developed for use in clinician and patient interviews.
Clinician Interview Results
Qualitative interviews were completed with a total of nine clinicians familiar with the treatment and management of patients with gMG, including seven neurologists (four of whom were from private practice), a neuromuscular specialist, and a physical therapist specializing in neurological conditions. Clinicians had between 6 and 33 years’ experience of treating patients with gMG and were currently seeing 3–20 patients per month; characteristics of the clinicians are summarized in Supplementary Material Table S4. Overall, there was no significant heterogeneity among the clinicians regarding their responses during interviews.
Clinician Feedback About Patients with gMG
All clinicians described patients with gMG as having a constellation of signs and symptoms affecting various muscle functions. Commonly noted signs and symptoms included general fatigue and muscle fatigability, ocular issues, difficulty swallowing, difficulty breathing, talking and speech difficulties, chewing difficulty and jaw weakness, muscle weakness of the upper and lower extremities, facial muscle weakness, and neck weakness. Clinicians noted that no two patients were the same, and the presenting signs and symptoms can vary greatly among patients, particularly if they are seen later in the day when muscle fatigability may be exacerbated. Clinicians noted that the type of muscle function impairment (single or multiple function impairment) and severity are factors that they consider when assessing the overall disease severity and developing treatment plans. To assess disease progression, clinicians compare assessments longitudinally while asking about frequency, resistance, timeframe, duration, and speed of changes (e.g., of muscle functions).
Clinician’s Description of the Signs, Symptoms, and Impacts of gMG and Their Clinical Assessment
For each of the major signs and symptoms of gMG, a brief overview of the clinicians’ descriptions and assessment in patients is given below.
Muscle weakness and fatigability were reported as the main characteristics of gMG. Clinicians noted that patients generally exhibit fatigue, an overall tiredness or general body weakness, and lack of energy, which is different to muscle fatiguability specifically caused by dysfunction of neuromuscular junctions [31].
Clinicians reported that ocular symptoms impact patients’ everyday activities in several ways, including their ability to read, watch television, and drive. Eye muscle weakness could also result in headache, light sensitivity, and nausea or vomiting in some patients.
Clinicians described how swallowing difficulties could be associated with choking episodes and in extreme cases, saliva aspiration issues could arise. They also reported that patients having difficulty swallowing often lose weight. Additionally, difficulty swallowing could cause coughing, which could lead to aspiration, which in turn puts patients at risk of infection.
Respiratory difficulties are one of the main symptoms clinicians look for when assessing MG. Breathing difficulties were noted to be a key sign of disease progression or worsening and could result in the need for a ventilator.
Changes in speech described by the clinicians included slurring, nasal voice/talking through the nose, lowered/weakened voice, and slower speed when speaking. Clinicians noted that this was a symptom they expected to worsen as the disease progressed. They also described the connection between speech difficulties and breathing difficulties.
Clinicians reported that muscle weakness of upper and lower extremities can have profound effects on patients’ lives, including difficulty walking, impairment of balance, washing or managing hair, lifting objects, and, in severe cases, paralysis.
Other signs, symptoms, and assessments included chewing difficulties, jaw and facial muscle weakness, and neck weakness.
Cognitive Debriefing of COAs
Clinicians were asked to debrief the items on the MG-ADL, MGC (round 1 and 2 interviews) and QMG (round 2 interviews only due to time constraints). For the MG-ADL, the focus was on item relevance, appropriateness of the recall period and response options for use in a clinical trial, and what would represent a clinically meaningful change for patients on the MG-ADL items. For the MGC, the focus was on items relating to patient history, as there was overlap with the MG-ADL and QMG; for the QMG, the focus was on the practical elements of performing each test item based on clinical experience.
All the MG-ADL items (talking, chewing, swallowing, breathing, impairment of ability to brush teeth or comb hair, impairment of ability to arise from chair, double vision, and drooping eyelid) and their respective response options were found to be relevant and understandable to patients per four of the five clinicians’ feedback. Clinicians offered slight modifications to select items to enhance clinical assessment, specifically regarding swallowing, getting up from a chair, and double vision. Furthermore, clinicians suggested follow-up questions for speech and fatigue. All clinicians who were asked about the recall period for MG-ADL indicated the 7-day period was appropriate for patients. Some clinicians suggested that as a result of factors such as medication usage and variation in symptom control, a longer recall period may be helpful.
Clinicians were asked to describe what they thought would be a clinically meaningful improvement or worsening on each of the MG-ADL items. Overall, a 1-point change for most of the items was found to be meaningful in either direction (improvement or worsening). There were, however, some instances in which one clinician described a 1-point change differently depending on the concept being measured. For example, a 1-point worsening on “shortness of breath” was described as concerning from a clinical perspective because of its importance for breathing and talking. Conversely, a 1-point worsening on “combing hair/brushing teeth” was considered less important, while a 2-point change on this item would be indicative of a greater impact on patients’ lives.
In round 1, the MGC speech, chewing, and swallowing items overlap with respective MG-ADL items. Round 2 interviews focused on the remaining MGC items, based on physical examination of the patient (ptosis, neck flexion, shoulder abduction, and hip flexion). All clinicians reported that a change in one category on each of the items of the MGC corresponded to a clinically meaningful improvement or worsening, which would aid assessment of patients’ signs, symptoms, and impacts over time, thereby allowing for treatment modifications when necessary.
Clinicians in round 2 interviews gave their perspectives on the QMG facial muscles, arm outstretched (90° sitting), forced vital capacity, hand grip, and leg outstretched (45° supine) items based on clinical practice experience. Clinicians noted that comorbidities, specifically orthopedic issues, could potentially complicate arm outstretched and hand grip assessments. Furthermore, accurately assessing forced vital capacity could be challenging because of facial muscle weakness.
Patient Results
The first round of patient interviews (n = 12) included a comprehensive CE section to fully capture the patient experience of gMG, specifically the signs, symptoms, and impacts of the disease. The second round of interviews (n = 12) focused on the relevance of the MG-ADL, QMG, and MGC. The sociodemographic and clinical characteristics of patients with gMG who participated in round 1 and round 2 interviews are presented in Tables 1 and 2, respectively.
Signs and Symptoms
In total, 29 unique signs and symptoms were reported during round 1 interviews, five of which were newly identified (i.e., not reported in the initial literature review; Supplementary Material Table S5). Saturation was achieved, with no new sign and symptom concepts emerging in the third group of round 1 interviews when compared to the first two groups within round 1 interviews (Supplementary Material Table S6).
During round 2 patient interviews, 37 signs and symptoms were reported spontaneously. Overall, the findings from the second set of interviews confirmed the CE findings of the first set. In addition, noise sensitivity and light sensitivity emerged as new concepts (each reported by one patient). During the second round of patient interviews, most patients considered muscle weakness of the legs and arms as their most bothersome symptom (n = 7), while general fatigue (n = 4), double vision (n = 4), neck weakness (n = 3), and shortness of breath (n = 3) were also reported as the most bothersome symptom.
Signs and symptoms identified as salient (defined in the “Salience Analysis”) were shortness of breath, general fatigue, muscle weakness of arms, legs, and neck, poor voice quality/losing voice (dysphonia), speech impairment (dysarthria), trouble swallowing liquids, choking, and environmental temperature heat sensitivity (Fig. 1).
Symptom saliency from round 1 patient interviews (N = 12). Bothersome ratings are based on the number of patients who provided a rating, which is not always the same as the number of patients who endorsed the concept. As a result of the iterative process during the interviews, some concepts were not explored with all the patients but were discussed later in the process, thus with fewer patients. Additionally, some patients provided qualitative descriptions and even with gentle encouragement by the interviewer did not provide a quantitative number. Therefore, not all symptom concepts were able to be included in this saliency grid. There are five symptom concepts for which no bothersome rating was provided, and which do not appear in this graph, including fatigability upon exertion, fatigability progression throughout the day, trouble aspirating saliva, sleep apnea, and cognitive impairment. The most salient signs and symptoms identified by patients are shown within the box. GI gastrointestinal
Key illustrative patient quotes for the most salient signs and symptoms are given in Table 3.
Impacts
During round 1 interviews, patients identified 50 unique impacts that were later divided by the researchers into nine domains, namely emotional, psychological, daily activities, physical function, self-care, eating, professional, social, and burden. Twenty-four of these impacts were newly identified (i.e., not reported in the initial literature review, Supplementary Material Table S7).
Concepts were distributed into “immediate impacts” (direct consequences of disease signs and symptoms) and “general impacts” (consequences of immediate impacts).
During round 1 interviews, identified salient impacts were work life, depression, difficulty walking, difficulty grooming hair, difficulty showering, difficulty brushing teeth, eating, personal relationships, family life, and participating in social activities (Fig. 2).
Impact saliency from round 1 patient interviews (N = 12). Disturbance ratings are based on the number of patients who provided a rating, which is not always the same as the number of patients who endorsed the concept. As a result of the iterative process during the interviews, some concepts were not explored with all the patients but were discussed later in the process, thus with fewer patients. Additionally, some patients provided qualitative descriptions and even with gentle encouragement by the interviewer did not provide a quantitative number. Therefore, not all impact concepts were able to be included in this saliency grid. There are two impact concepts for which no disturbance rating was provided, and which do not appear in this graph, including stress and feeling upset about the disease. The most salient impacts identified by patients are shown within the box
Saturation was reached for most impacts; however, there were a few concepts that emerged during the third group of interviews with four patients (anger, loss of identity, hot baths, physical yard labor, and hand grip). Upon reviewing concepts closer, two were merged with other impacts previously identified (anger with irritation; physical yard labor with exercise). As loss of identity and hot baths were distal impacts mentioned by one and two patients, respectively, no further interviews were conducted.
During the second round of patient interviews, 52 impacts were spontaneously mentioned, of which 13 were not previously reported in interviews or literature, namely difficulty with writing, unable to sit upright, difficulty getting to the bathroom, difficulty bending down, education, perceived as disinterested by others, difficulty going outside, difficulty singing, cutting nails, shaving, weight gain, unable to play video games, and other people frustrated with them due to disease.
Key illustrative patient quotes for the most salient impacts are summarized in Table 4.
Cognitive Debriefing of COAs
Specific aspects of the COAs assessed during patient interviews included (1) relevance of the concepts covered both by the muscle function impairments and by the response options, (2) the appropriateness of the recall period of the COAs, and (3) the ecological validity (i.e., the degree to which scores of the COA reflect experience in the natural environment), which was assessed by asking the patients the link between the situation involving the muscle function impairment assessed and the associated response options covered by the COAs to their everyday experience with this situation.
Responses from patients suggest that the items on the MG-ADL, MGC, and QMG are all capturing concepts that are important and relevant to them, generally capturing their real-world experiences of living with gMG. Patients suggested additional items to the MG-ADL to capture signs and symptoms they experience that are not asked about in the instrument (e.g., walking, cognitive functioning, having a shower, blurry vision and headaches, overall fatigue, lifting arms in front of the patient, arm strength, neck extension, a visual assessment of the face, measurement of mucus/secretion pooling at waking, and walking on tiptoes/heels). With these exceptions related to performance-based instruments, patients thought all three COAs generally reflected their day-to-day experience. Some patients did not consider performance-based instruments sufficient to capture the full extent of the severity of their signs and symptoms, particularly strength-based instruments; however, these were described as somewhat difficult to reliably capture and accurately measure the real-world lived experience.
Conceptual Model of gMG
Findings from all clinician interviews, patient interviews, and previous qualitative literature, including a recently published conceptual model, were combined [32]. The final model is a comprehensive representation of signs, symptoms, and impacts experienced by people living with gMG (Fig. 3).
Conceptual model of patient sign, symptom, and impact experience with gMG. CE concept elicitation gMG generalized myasthenia gravis MG-ADL Myasthenia Gravis-Activities of Daily Living, QMG Quantitative Myasthenia Gravis, MGC Myasthenia Gravis Composite, MG-QoL-15R Myasthenia Gravis Quality of Life 15-item Scale-Revised, Neuro-QoL-F Neurology Quality of Life Fatigue-short form
Discussion
Literature published prior to the initiation of this work describing the signs, symptoms, and impacts experienced by adult patients with gMG was limited. This qualitative study provides a robust and holistic picture of the signs, symptoms, and impacts that characterizes gMG from both a clinician and patient perspective more completely than was previously understood. gMG has a detrimental impact on many domains of patients’ lives, which expands on previous understanding, as demonstrated by the conceptual model (Fig. 3), results for which are substantiated by other qualitative investigations conducted in parallel to the current study.
Among the signs and symptoms identified, muscle weakness that affects several muscle functions (bulbar, ocular, respiratory, and upper and lower extremities), and the fatigability upon exertion and/or as the day progresses, are the main characteristics of gMG. Importantly, this study identified seven new signs and symptoms and 37 new impacts of gMG that had not been previously reported (Supplementary Material Tables S5, S6, and S7).
The most salient signs and symptoms identified from patient interviews were shortness of breath, general fatigue, muscle weakness of arms, legs, and neck, poor voice quality/losing voice, speech impairment, trouble swallowing liquids, choking, and heat sensitivity. At the time of the interviews, all patients were receiving treatment for their gMG and rated their experiences on the basis of their current condition; therefore, these findings highlight the persistent nature and continuing unmet need of gMG irrespective of treatment. Symptoms of light sensitivity and noise sensitivity were also reported by the sample. Light sensitivity was mentioned by one clinician as well in the context of manifestations of impacted ocular functioning due to gMG. Further to this, patients and clinicians reported gastrointestinal symptoms, and patients reported new symptoms such as sleep apnea, stiffness, and heat sensitivity which may or may not be attributable to impaired neuromuscular transmission seen in gMG. Importantly, gastrointestinal symptoms are also reported as adverse events experienced by patients receiving treatment with acetylcholinesterase inhibitors. All experiences reported by patients are given due consideration in the conceptual model.
The MG-ADL, MGC, and QMG are widely used questionnaires in gMG that can be used to aid clinical assessment of patients’ signs, symptoms, and impacts. All the muscle function impairments assessed by the MG-ADL, MGC, and QMG were found to be relevant and appropriate to patients’ experience of living with gMG and reflective of the physical functions and related activities of patients’ everyday life that are impacted by the disease. The limitations in daily activities and the attributes (e.g., frequency, severity) associated with each MG-ADL item also aligned with the clinicians’ understanding of the impacts of gMG signs and symptoms. While this study found that these specific COAs are not used routinely in the clinical practice of the study clinicians, the items that comprise all three COAs are reflective of how clinicians assess disease progression and treatment benefit, and facilitate decision-making around treatment planning.
Across all COA assessments, the importance of fatigability on the assessment of gMG-related signs and symptoms was highlighted by both clinicians and patients; it is therefore recommended that patients are assessed at the same time of day and at the same interval from visit to visit in clinical practice to get a clear picture of other signs, symptoms, and impacts outside of fatigability, as this will aid in minimizing variability due to the increase in fatigability throughout the day that patients often experience as a result of their gMG.
The majority of physical signs and symptoms related to muscle function impairments are covered when the three COAs are administered in concert, indicating good conceptual coverage of physical signs and symptoms related to muscle function impairment, which provides a comprehensive picture of gMG from the perspective of the patient (MG-ADL), substantiated with tests of patients’ performance (QMG, MGC). Five of the 14 physical function impacts mentioned in the CE interviews are included (difficulty keeping the arm lifted, difficulty gripping objects, holding up the head, difficulty bending down, and getting out of a chair). Emotional, social, psychosocial, coping, cognitive functions, or other distal impacts from the conceptual model are also not captured in the three COAs, although these are more accurately assessed by patients themselves.
The patient population in this study was sufficient to meet the objectives; concept saturation was reached at the third round of interviews. It was diverse in terms of age (30–75 years old), time since diagnosis (less than a year to over 25 years), and education levels, thereby supporting the representativeness of these data to the wider gMG population. However, it was limited by geographic location (within the USA) and the inclusion requirement for participants to be fluent in English. Further research could explore the patient experience in the rest of the world to understand linguistic and cross-cultural nuances. Although there are diversity challenges for the research of rare diseases, a more inclusive patient study could analyze the lived experience of gMG further.
Importantly, determination of what the optimal outcomes measurement strategies are within gMG continues to evolve, including new consensus recommendations for addressing variability in gMG clinical trials [33] as well as development of de novo patient-reported outcome domains to assess gMG symptoms from the patient perspective [32, 34]. While the current research focuses on instruments widely considered relevant assessments of the key aspects of gMG, assessment of the patient experience with other outcomes instruments, such as the MG-QoL-15R and Neuro-QoL Fatigue, may allow coverage of immediate and general impacts experienced by patients and could be used as part of a holistic and patient-centric measurement strategy (illustrated in Fig. 3).
Conclusion
This research emphasizes the voice of the patient, including a conceptual model of gMG highlighting the wide range of symptoms and impacts that adult patients with gMG experience in their everyday lives. As treated patients were involved in this research, this highlights an unmet need from the patient perspective as well as those aspects most relevant and bothersome to patients with gMG not previously identified in the literature review. As the MG landscape continues to evolve with respect to best means of assessing the patient experience, these findings may aid in informing a patient-centric and holistic measurement strategy within future research, clinical practice, and/or patient interventions.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Howard JF Jr. Clinical overview of MG. 2015. https://myasthenia.org/Professionals/Clinical-Overview-of-MG. Accessed Oct 1, 2022.
Gilhus NE, Tzartos S, Evoli A, et al. Myasthenia gravis. Nat Rev Dis Primers. 2019;5(1):30.
Gilhus NE. Myasthenia gravis. N Engl J Med. 2016;375(26):2570–81.
Narayanaswami P, Sanders DB, Wolfe G, et al. International consensus guidance for management of myasthenia gravis: 2020 update. Neurology. 2021;96(3):114–22.
Barnett C, Bril V, Kapral M, et al. A conceptual framework for evaluating impairments in myasthenia gravis. PLoS ONE. 2014;9(5):e98089.
Chen YT, Shih FJ, Hayter M, et al. Experiences of living with myasthenia gravis: a qualitative study with Taiwanese people. J Neurosci Nurs. 2013;45(2):E3–10.
Raggi A, Schiavolin S, Leonardi M, et al. Development of the MG-DIS: an ICF-based disability assessment instrument for myasthenia gravis. Disabil Rehabil. 2014;36(7):546–55.
Richards HS, Jenkinson E, Rumsey N, et al. The psychosocial impact of ptosis as a symptom of myasthenia gravis: a qualitative study. Orbit. 2014;33(4):263–9.
Farrugia ME, Goodfellow JA. A practical approach to managing patients with myasthenia gravis-opinions and a review of the literature. Front Neurol. 2020;11:604.
Barnett C, Bril V, Kapral M, et al. Development and validation of the myasthenia gravis impairment index. Neurology. 2016;87(9):879–86.
Kabasawa C, Shimizu Y, Suzuki S, et al. Taste disorders in myasthenia gravis: a multicenter cooperative study. Eur J Neurol. 2013;20(1):205–7.
Jackson K, Parthan A, Lauher-Charest M, et al. Understanding the symptom burden and impact of myasthenia gravis from the patient’s perspective: a qualitative study. Neurol Ther. 2023;12(1):107–28.
US Food and Drug Administration. Patient-focused drug development: collecting comprehensive and representative input. Guidance for industry, Food and Drug Administration staff, and other stakeholders. 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-methods-identify-what-important-patients. Accessed Jan 11, 2023.
US Food and Drug Administration. The 21st Century Cures Act. 2016. https://www.fda.gov/regulatory-information/selected-amendments-fdc-act/21st-century-cures-act. Accessed Jan 11, 2023,
US Food and Drug Administration. Patient-focused drug development: methods to identify what is important to patients. Guidance for industry, Food and Drug Administration staff, and other stakeholders. 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-methods-identify-what-important-patients. Accessed Jan 11, 2023.
Ciani O, Jommi C. The role of health technology assessment bodies in shaping drug development. Drug Des Dev Ther. 2014;8:2273–81.
De Sutter E, Borry P, Huys I, et al. Informing a European guidance framework on electronic informed consent in clinical research: a qualitative study. BMC Health Serv Res. 2023;23(1):181.
Yang RR. A patient advocating for transparent science in rare disease research. Orphanet J Rare Dis. 2023;18(1):14.
Committee for Medicinal Products for Human Use. Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. London: European Medicines Agency; 2005.
US Department of Health and Human Services FDA Center for Drug Evaluation and Research, US Department of Health and Human Services FDA Center for Biologics Evaluation and Research, US Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4(1):79.
US Food and Drug Administration. Guidance document: Patient-focused drug development: Selecting, developing, or modifying fit-for-purpose clinical outcome assessments. 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-selecting-developing-or-modifying-fit-purpose-clinical-outcome. Accessed Oct 1, 2022.
Wolfe GI, Herbelin L, Nations SP, et al. Myasthenia gravis activities of daily living profile. Neurology. 1999;52(7):1487–9.
Barohn RJ, McIntire D, Herbelin L, et al. Reliability testing of the quantitative myasthenia gravis score. Ann N Y Acad Sci. 1998;841:769–72.
Burns TM, Conaway MR, Cutter GR, et al. Construction of an efficient evaluative instrument for myasthenia gravis: the MG composite. Muscle Nerve. 2008;38(6):1553–62.
Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1—eliciting concepts for a new PRO instrument. Value Health. 2011;14(8):967–77.
Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2—assessing respondent understanding. Value Health. 2011;14(8):978–88.
Jaretzki A 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology. 2000;55(1):16–23.
Bryman A, Burgess B. Analyzing qualitative data. 1st ed. London: Routledge; 1994.
Thomas DR. A general inductive approach for analyzing qualitative evaluation data. Am J Eval. 2006;27(2):237–46.
Law N, Davio K, Blunck M, et al. The lived experience of myasthenia gravis: a patient-led analysis. Neurol Ther. 2021;10(2):1103–25.
Ruiter AM, Verschuuren J, Tannemaat MR. Fatigue in patients with myasthenia gravis. A systematic review of the literature. Neuromuscul Disord. 2020;30(8):631–9.
Cleanthous S, Mork AC, Regnault A, et al. Development of the Myasthenia Gravis (MG) Symptoms PRO: a case study of a patient-centred outcome measure in rare disease. Orphanet J Rare Dis. 2021;16(1):457.
Guptill JT, Benatar M, Granit V, et al. Addressing outcome measure variability in myasthenia gravis clinical trials. Neurology. 2023. https://doi.org/10.1212/WNL.0000000000207278.
Regnault A, Morel T, de la Loge C, et al. Measuring overall severity of myasthenia gravis (MG): evidence for the added value of the MG symptoms PRO. Neurol Ther. 2023. https://doi.org/10.1007/s40120-023-00464-x.
Acknowledgements
The authors would like to thank patients and their families who were involved in this study. The authors thank Yi Zhang for early contributions to the development of this publication, and Richa Attre from Regeneron Pharmaceuticals, Inc. for her excellent assistance with development of the manuscript.
Medical Writing/Editorial Assistance
Medical writing support was provided by Rachel McGrandle, MSc, of Prime, Knutsford, supported by Regeneron Pharmaceuticals, Inc., according to Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M22-1460).
Funding
This study, including the Rapid Service Fee for Neurology and Therapy, was funded by Regeneron Pharmaceuticals, Inc. The sponsor was involved in the study design, interpretation of data, and data checking of information provided in the manuscript. IQVIA was involved in the study design and collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript.
Author information
Authors and Affiliations
Contributions
Christopher Hartford contributed to conceptualization, methodology, project supervision, visualization, and writing of study report (original draft, review, and editing). Steven Sherman contributed to conceptualization, funding acquisition, methodology, project supervision, visualization, and writing of study report (original draft, review, and editing). Stella Karantzoulis contributed to data curation, methodology, formal analysis, overall project supervision and management, visualization, and writing of study report (original draft, review, and editing). Isabelle Guillemin contributed to data curation, methodology, formal analysis, supervision, visualization, and writing of study report (original draft, review, and editing). Michael Phinney contributed to project administration, data curation, visualization, and writing of study report (original draft, review, and editing). Kimberly Kelly contributed to interview conduct, data curation, methodology, formal analysis, supervision, visualization, and writing of study report (original draft, review, and editing). Kayla Negron contributed to data curation, formal analysis, visualization, and writing of study report (review and editing). Shruti Raja contributed to interpretation of study results and writing of study report (review and editing). Diana Rofail contributed to conceptualization, funding acquisition, methodology, project supervision, visualization, and writing of study report (original draft, review, and editing). The authors were responsible for all content and editorial decisions.
Corresponding author
Ethics declarations
Conflict of Interest
Christopher Hartford, Steven Sherman, and Diana Rofail are all employees of and stockholders in Regeneron Pharmaceuticals, Inc. Stella Karantzoulis, Isabelle Guillemin, Michael Phinney, Kimberly Kelly, and Kayla Negron were all employees of IQVIA at the time of study conduct. Shruti Raja is a consultant to Regeneron Pharmaceuticals, Inc. The authors received no honoraria related to the development of this publication.
Compliance with Ethics Guidelines
The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and are consistent with Good Clinical Practice and applicable regulatory requirements. The study was reviewed and approved by the WCG institutional review board (IRB), IRB number 20213567. The research protocol, interview guides, all documents associated with patient interviews, and all clinician communication documents were also reviewed and approved by WCG IRB. Informed, written consent was obtained electronically from each participant prior to their enrollment.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hartford, C.A., Sherman, S.A., Karantzoulis, S. et al. Experience of Daily Life with Generalized Myasthenia Gravis: A Qualitative Investigation and Assessment of Instrument Content Validity. Neurol Ther 12, 2079–2099 (2023). https://doi.org/10.1007/s40120-023-00544-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00544-y