Abstract
Motor fluctuations (MF) are deemed by patients with Parkinson's disease (PD) as the most troublesome disease feature resulting from the increasing impairment in responsiveness to dopaminergic drug treatments. MF are characterized by the loss of a stable response to levodopa over the nychthemeron with the reappearance of motor (and non-motor) parkinsonian clinical signs at various moments during the day and night. They normally appear after a few years of levodopa treatment and with a variable, though overall increasing severity, over the disease course. The armamentarium of first-line treatment options has widened in the last decade with new once-a-daily compounds, including a catechol O-methyltransferase inhibitor – Opicapone-, two MAO-B inhibitors plus channel blocker – Zonisamide and Safinamide and one amantadine extended-release formulation – ADS5012. In addition to apomorphine injection or oral levodopa dispersible tablets, which have been available for a long time, new on-demand therapies such as apomorphine sublingual or levodopa inhaled formulations have recently shown efficacy as rescue therapies for Off-time treatment. When the management of MF becomes difficult in spite of oral/on-demand options, more complex therapies should be considered, including surgical, i.e. deep brain stimulation, or device-aided therapies with pump systems delivering continuous subcutaneous or intestinal levodopa or subcutaneous apomorphine formulation. Older and less commonly used ablative techniques (radiofrequency pallidotomy) may also be effective while there is still scarce data regarding Off-time reduction using a new lesional approach, i.e. magnetic resonance-guided focused ultrasound. The choice between the different advanced therapies options is a shared decision that should consider physician opinion on contraindication/main target symptom, patients’ preference, caregiver’s availability together with public health systems and socio-economic environment. The choice of the right/first add-on treatment is still a matter of debate as well as the proper time for an advanced therapy to be considered. In this narrative review, we discuss all the above cited aspects of MF in patients with PD, including their phenomenology, management, by means of pharmacological and advanced therapies, on-going clinical trials and future research and treatment perspectives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
• Motor fluctuations (MFs) are deemed by patients with Parkinson’s disease (PD) as the most troublesome disease feature. |
• In this narrative review, we discuss MF phenomenology and management, by means of pharmacological and advanced therapies, touching upon on-going clinical trials, future research, and treatment perspectives. |
• Oral treatment for MF reposes on levodopa adjustments, including the IR and ER formulations and wide armamentarium of add-on and on-demand therapies whose choice is based on patients’ age, cognitive status, previous and on-going treatments, considering their different effectiveness, tolerability profile and ease of use. |
• Advanced treatments for MF include surgical (neuromodulation or lesional approch) and device-aided therapies (DAT), that can be proposed only to sub-set of advanced PD patients, based on patients’ preferences, caregivers availability and clinical contraindications. |
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder, with an estimated 6 million people affected worldwide. PD prevalence is expected to further increase by two- to threefold until 2040, following the trend of recent decades [1].
PD treatment is based on symptomatic pharmacological and non-pharmacological options, with levodopa (LD) being the main stain of the pharmacological armamentarium [2, 3]. Nevertheless, after a few or several years of LD treatment, most patients with PD experience motor complications (MC), including mainly wearing-off (end-of-dose deterioration) and peak-dose dyskinesias, with a variable but usually consistent impact on activities of daily living (ADLs) [4, 5]. Ultimately, troublesome MC may cause extreme fluctuations of the motor state of patients, representing one of the main sources of patients’ disability and impaired quality of life (QoL). Of note, wearing-off is indicated by patients with PD as the most disabling motor symptom, even worse than dyskinesia [6, 7].
Risks factor for MC development include a variable list of phenotypic, clinical, genetic and pharmacologic factors, such as longer disease duration, younger age at onset, being carriers of genetic mutations such as parkin, PINK1, and DJ-1, higher LD cumulative dose, female gender, and low body weight [5, 8].
From a pathophysiological point of view, intermittent oral delivery of levodopa, as opposed to continuous physiological dopaminergic stimulation, along with delayed or erratic gastric emptying and the relentless loss of nigrostriatal nerve terminals and reduced endogenous dopamine storage/release capacity, contribute to the appearance of MC, with symptomatic benefit becoming progressively dependent on oral levodopa intake and plasma bioavailability [9].
Nowadays, a wide armamentarium of oral and second-line treatments is available for the management of motor fluctuations (MF), including some new compounds approved over the last decade, the appearance of a few new rescue therapies and a new formulation of subcutaneous levodopa, which is still waiting for market approval.
In this narrative review, we expressively focus on MF, i.e. Off-time, of patients with PD, discussing their phenomenology, management, by means of pharmacological and advanced therapies, on-going clinical trials and future research and treatment perspectives.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Motor Fluctuations: Epidemiology and Phenomenology
It has been estimated that, after initiation of dopaminergic treatment, on average 10% of patients per year will developed motor complications. Previous works have found a prevalence of around 50% of MF after 5 years of disease, although a prevalence as high as 88% and as low as 20% have also been reported [10, 11]. MF were initially thought to be advanced-disease features, occurring in patients with long-disease duration. However, it has been reported that up to 50% of patients may have the onset of MF within 2 years of starting LD therapy [5]. In addition, in the ELLDOPA trial, by the end of the 9-month trial period, almost a third (29.7%) of patients receiving the highest daily dose of levodopa (600 mg/day) experienced wearing-off [12].
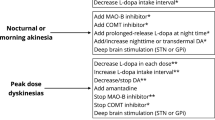
At the beginning of their appearance, MF usually have a somehow recurrent, predictable pattern, appearing in a time-dependent manner from the last levodopa intake (initially 4 h from the last intake). Such end-of-dose phenomena have been described as the most common, first-presentation form of MF, along with the presence of PD symptoms in the morning before the first dose of levodopa (‘morning akinesia’), occurring among 54–70% patients with PD [13] (Fig. 1). Nocturnal akinesia is associated with worst sleep quality, decreased sleep efficiency, and decreased QoL, often accompanied by a painful posture, generally on the lower limb-off-dystonia [14]. As highlighted by the CALM-PD study, the wearing-off phenomenon may not be only the first MC to occur but could also be a predictor for the development of dyskinesia, as MF and dyskinesias appear to be interrelated, with the presence of one associated with the earlier development of the other [15]. This may indicate that the emergence of the wearing-off may sign the beginning of a more complex, advanced phase of the disease [10, 16]. With disease progression, MF become more and more intense and unpredictable, widening the phenotype of possible MF. On–off phenomenon may then arise, and represent rapid switches between the On and Off states, along with sudden-Off, seemingly without obvious relationship to levodopa dosing [13]. Patients can notice an increased latency after LD intake for the beginning of the clinical benefit (delayed-On) (Fig. 1), even after the first-day dose (“prolonged morning akinesia”) [14, 17] or a complete failure to respond to a LD dose (No-On) [13]. More rarely, patients present a transient worsening of symptoms at the beginning of LD dose, often related to delay in gastric emptying, often presenting as an increase in tremor (“beginning of dose worsening”) or a random “yo-yoing” effect switching from being On with dyskinesia to Off and then to On again in a rapid and unpredictable way [13].
Despite MF being more commonly reported and recognized both by patients and clinicians, the re-emergence of non-motor symptoms (NMS) before each LD intake, can also occur and even precede the emergence of the motor wearing-off phenomenon [18]. Non-motor fluctuation (NMF) are often underestimated and their recognition is facilitated if tagged along with MF. Large cross-sectional studies have shown that 60–100% of patients with PD report NMF, appearing to have an impact on patient independence and QoL possibly even greater than MF [18, 19]. Pain, diffuse or restricted to a body part, and mood symptoms are the NMF with the greatest impact on QoL. Other NMF may occur, including autonomic ones, i.e. thoracic and abdominal pain, possibly mimicking medical emergencies, dyspnea, constipation, urinary urgency diaphoresis and visual complaints, neuropsychiatric fluctuations, such as forgetfulness, slowness of thinking, attention problems, anxiety, depression, irritability, fatigue, apathy, mutism and hallucinations, and others such as akathisia and mutism [19].
MF pathophysiology is strictly related to the pharmacokinetics and pharmacodynamics of LD which are dominated by two features: the short plasma half-life of the drug and the short-duration response which represents the portion of the antiparkinsonian response that parallels the plasma LD levels [11]. These pharmacological features are the basis of MF. Concomitantly, LD treatment induce a long-duration response that builds up over weeks and likewise dissipates slowly. The long-duration response may partially account for the absence of MF in early patients with PD treated with LD [20]. Numerous pharmacological efforts have been made to overcome the limitations related to LD short half-life, to increase the long-duration response, aiming to smooth LD-related motor complications and extend its effect, and to limit the issues related to the oral route and intestinal absorption of LD, that, later in the disease, may render this route lengthy and inefficacious. These efforts include the use of LD add-on strategies and the development of newer apomorphine and LD formulations, including extended release (ER) formulations or infusion ones [21].
Oral Treatment Options
Oral treatment options for motor fluctuations include optimization of levodopa dosage and frequency of administration, change of levodopa formulation and the use of adjunct therapies, classically including dopamine agonists (DAAs), monoamine oxidase type-B inhibitors (MAO-B Is) and COMT inhibitors (COMT-Is) [2, 22]. More recently, a formulation of amantadine ER (ADS-5012) [23] and a channel blocker, i.e. Adenosine A2A receptor antagonists (AA2AA), have also shown their efficacy for MF treatment [24, 25].
Levodopa
Overview
LD administration can be increased in terms of dose at each intake if wearing-off is the main symptom, or frequency, if dyskinesia are also present [22].
There is no evidence to switch from IR to controlled-release (CR) formulations in case of diurnal MF, while CR is usually indicated at bedtime for night-time or morning akinesia [22]. LD is also available in dispersible preparations, which are designed to have faster onset of symptomatic effect (see “On-Demand” section).
In the last decade, three new ER LD formulations have been investigated for the treatment of MF, with positive results for two of them, IPX066 and IPX203 [26, 27]. Conversely, results were inconsistent for the Accordion Pill®, a gastric-retention oral delivery platform based on folded multilayer films, with an initial positive Phase II [28], but followed by a negative Phase III with consequent development interruption.
So far, IPX066 (Rytary®) is the only marketed one. It is a multiparticulate, ER formulation of carbidopa and LD in a 1:4 ratio [29, 30]. This compound, which is approved only in the United States (US), is usually administered t.i.d., and is designed to dissolve at different rates to ensure the release and absorption of LD over a longer time frame than that provided by standard LD, with consequent dosing intervals of approximately 6 h. The recommended starting dosage of IPX066 naïve patients is 23.75 mg/95 mg taken orally three times a day up to a maximum recommended dose of 97.5 mg/390 mg taken three times.
IPX203 is a multiparticulate oral capsule formulation of CD/LD (ratio 1:4), which has been specifically designed to provide the desired LD plasma profile of a rapid initial rise in plasma LD followed by prolonged, steady concentrations that extend beyond currently available formulations [27]. Indeed, after a single dose of IPX203, LD concentrations were sustained above 50% of peak concentration for 4.6 h versus 1.5 h for IR carbidopa-LD.
Efficacy on Off-time Reduction
There is no randomized clinical trial (RCT) that has investigated the effect of LD/carbidopa IR dose/intake adaptations on Off-time reduction versus placebo. Even if not specifically designed to evaluate the effect of oral LD adaptations, we can infer its effect on Off-time reduction by looking at the data of RCTs comparing device-aided therapies/surgical therapies versus oral treatment. Most of the trials adopted an open-label design and showed some benefit on MF when carefully adjusting the dose/intake regimen, also including other oral add-on therapies, although it could also be related to the fact that patients are more adherent in such trial conditions. At the same time, looking at the double-blind double-dummy trail comparing standard LD/carbidopa versus levodopa/carbidopa intestinal gel (LCIG), a reduction of − 2.14 (0.66) h/day over 12 weeks [31] was observed for oral treatment. Additionally, two RCTs have shown the efficacy of IPX066 on Off-time reduction versus LD/carbidopa IR and versus LD/carbidopa plus entacapone (ENT), with a reduction of 1.17 h/day and about 1.4 h/day, respectively [32, 33].
Safety Data
Possible dose-dependent adverse events (AEs) of LD are well known, and classical dopaminergic includes gastrointestinal side effects, including nausea, vomiting, and constipation, which tend to decrease over time, or more rarely orthostatic hypotension (OH) and hallucinations, especially among elderly patients with cognitive impairment. Additionally, long-term LD use is associated with MC development, which increases at an estimated rate of 10% per year [34].
News
In a small Phase II, open-label, rater-blinded, multicenter, randomized crossover trial including 28 patients with PD, IPX203 showed a reduction of − 2.26 h/day (CI 95% − 3.17 to − 1.35) compared to standard LD, after 15 days of treatment [27]. Thereafter, two large Phase 3 trials (NCT03670953, on 631 patients; NCT03877510 on 420 patients) with positive results on good On-time increment, have been recently performed on IPX203 (RISE-PD trial). Preliminary public results (Congress communication) informed that IPX203 provided 1.55 more h/day of good On-time per dose and a reduction from baseline in Off-time of − 0.48 h/day (p = 0.0252) versus LD/carbidopa IR [35].
Add-on Oral Therapies
Overview
Dopamine Agonists
Dopamine agonists (DAAs) are divided into two classes: ergot-derived and nonergot-derived, both acting directly on striatal postsynaptic dopamine receptors. Due to drug-related AEs, including retroperitoneal, pleuropulmonar, and valvular heart fibrosis, the ergot-derived DAAs (bromocriptine, cabergoline, lisuride, dihydroergocryptine, and pergolide), are not used anymore [36]. Pramipexole (D2, D3 agonist), ropinirole (D2, D3, D4 agonist), piribedil (D2, D3 agonist and α2 antagonist), and rotigotine (D1, D2, D3 agonist), are nonergot-derived DAAs. All agonists must be progressively titrated to find the optimum dose and avoid AEs. Classical and older formulations are usually given t.i.d., al though more practical ER formulations taken once daily are available and more often used for ropinirole and pramipexole, as well as for rotigotine (marketed as transdermal formulations).
To date, DAAs, specifically pramipexole, ropinirole and cabergoline, are the only oral add-on therapies that have been shown to be efficacious to delay MF appearance, based on parallel group RCT versus LD [37].
Apomorphine, which is the oldest drug in this class, is an aporphine alkaloid derived from acidification of morphine. It is a nonselective agonist for dopamine D2 and, to a lesser extent, D1 receptors, but also functions as antagonist for α-adrenergic and 5-HT2 receptors [38]. Due to its limited oral bioavailability (< 4%), different parenteral administration routes have been applied and apomorphine is currently administered non-orally (via subcutaneous or sublingual routes) (see next paragraphs).
MAO-B inhibitors. These drugs are strong, selective MAO-B inhibitors (MAO-B Is) that reduce the catabolism of dopamine, thereby increasing the availability of this neurotransmitter at the synaptic level. There are two classical MAO-B Is: selegiline and rasagiline [39]. Selegiline was the first marketed MAO-B. It is administered at 5–10 mg, once daily; titration is not required. Rasagiline is given at a dose of 1 mg once daily (0.5 mg only approved in the US). In 2016, a new MAO-B I, safinamide, owing both dopaminergic properties, namely highly selective and reversible MAO-B I, and nondopamimetic properties, namely selective sodium channel blockade and calcium channel modulation, was introduced in Europe as an adjunct therapy to L-dopa [40]. Safinamide treatment is usually begun at the dose of 50 mg/day, once daily, with the possibility of further increase up to 100 mg, after a month.
Zonisamide is another MAO-B I which has multiple functions, including inhibition of sodium channels, T–type calcium channels, and striatal D1-receptor–associated gamma aminobutyric acid–ergic transmission, and activation of dopamine synthesis and dopamine release. Zonizamide 50 mg has been shown to be effective in reducing Off-time in a double-blind RCT, but, currently, approval for MF treatment has only been granted in Japan [41].
COMT-inhibitors. COMT is an enzyme that catalyzes the metabolism of levodopa to 3-O-methyldopa, prolonging the maintenance of serum levodopa levels and hence producing a longer and more stable clinical response [42]. There are three COMT-inhibitors (COMT-Is) approved for the treatment of end-of-dose MF in patients with PD: entacapone ENT, tolcapone (TOL) and opicapone (OPC) [42]. TOL, a potent and selective COMT-I, with both a central and peripheral effect, was the first COMT-I to be introduced in the market. Nevertheless, its practical utility is limited due to the risk of liver toxicity that implies mandatory repeated frequent liver function monitoring during the first 6 months of therapy [43]. ENT is a peripheral COMT-I, used as first-line strategy for MF management. It is given as an extra tablet with each L-dopa dose (maximum recommended dose in Europe is 200 mg ten times daily) or as triple fixed-dose combination of levodopa/carbidopa/entacapone (Stalevo®) [44]. OPC, approved in Europe in 2016 as LD add-on therapy for the treatment of end-of-dose MF, is a new long-acting, peripherally selective, COMT-I. OPC 50 mg should be taken once daily at bedtime, at least 1 h before or after LD combinations [45].
Adenosine A2A Antagonist. Over the last 15 years, several RCT showed the efficacy of Istradefylline, an adenosine A2A antagonist, in the reduction of Off-time [25, 46]. So far, Istradefylline is marketed only in Japan and US and administered at 20 mg or 40 mg, in a once-a-day intake in the morning.
Amantadine ER. In 2021, based on the post hoc analysis of the EASE-LID trials powered for dyskinesia reduction effect, the FDA has granted the approval of ADS-5012, a new formulation of amantadine ER, for the treatment of MF [23, 47]. ADS-5012 is administered once a day, at night, starting from the initial dose of 137 mg up to 234 mg/day.
Efficacy on Off-time Reduction
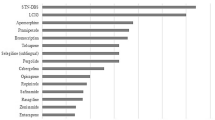
Effect size on Off-time for oral treatment are detailed in Table 1, while recommendations of the International Movement Disorder Society (MDS) for efficacy and clinical use of oral treatments for the management of MF are summarized on Table 4. Regarding effect size, in 2010, a Cochrane meta-analysis assessed, through an indirect comparison, the benefits and risks of the three main classes of drugs—DAAs, COMT-Is and MAO-B Is—used as adjuvant treatment for levodopa in patients with PD having motor complications [48]. Based on that Cochrane meta-analysis, DAAs seem slightly more efficacious when compared to COMT-Is and MAO-B Is. Indeed, when compared to placebo, the following values were met for each class: − 1.54 h/day for DAAs, − 0.83 h/day for COMT-Is and − 0.93 h/day for MAO-B Is [48]. However, the 2010 Cochrane meta-analysis did not include Safinamide, OPC, Zonisamide, Istradefylline and Amantadine ER, as they were not licensed at the time.
In a RCT Phase III OPC reached about 1 h/day of reduction compared to placebo, which was about 26.2 min/day more of the reduction obtained for ENT in the same trial, resulting in a proof of superiority versus placebo and non-inferiority versus ENT [49]. Open-label extension of the first double-blind trial showed that such an effect on Off-time reduction was maintained up to 1 year [50], and that patients who switched from ENT to OPC 50 mg had a further decrease in Off-time of about − 39.3 min/day [51]. Of note, a recent pharmacokinetic study, evaluated the effect of OPC 50 mg on LD bioavailability when switching from LD/carbidopa 500/125 mg (five intakes) to LD/carbidopa 400/100 mg given over either four or five intakes/day + OPC 50 mg. The addition of OPC, in spite of 100 mg LD reduction, allowed a higher levodopa bioavailability with avoidance of trough levels, with a reduction in 24 h Off-time of − 42.5 min and − 93.3 min, if LD was taken in four or five intakes, respectively [52]
Regarding Safinamide, a Phase III double-blind placebo-controlled RCT of 24 weeks showed a reduction of about 0.62 h/day of Off-time versus placebo [53]. The effect increased up to 0.86 h/day, with safinamide 100 mg when analyzing pooled data of two RCTs by means of a post hoc analysis, without aggravation of dyskinesia [53,54,55]. The efficacy of Zonisamide 50 mg has been also proved in a large Phase III trial against placebo over 12 weeks, showing a reduction of Off-time of − 0.719 h/day [95% CI − 1.198, 0.219, p < 0.005) versus placebo [41]. Zonisamide effect on Off-time reduction was dose dependent as 25 mg obtained a lower efficacy with − 0.436 ± 0.176 h/day (not versus placebo that obtained a reduction of − 0.011 ± 0.176 h/day) [41].
As said, the efficacy of Amantadine ER (ADS-5012) on Off-time reduction has been only evaluated by means of post hoc analysis, pooling the data of two Phase III RCTs (NCT02136914, NCT02274766). After 12 weeks of treatment, mean placebo-subtracted treatment difference in Off-time was − 1.00 [− 1.57, − 0.44] h/day[23]. Stratifying the population by baseline Off-time of ≥ 2.5 h/day the reduction in Off-time was − 1.2 [− 2.08, − 0.32] h in the ≥ 2.5 h subgroup (n = 102) and − 0.77 [− 1.49, − 0.06] in the < 2.5 h subgroup (n = 94) [23].
Finally, regarding istradefylline, the results on Off-time reduction are conflicting. Eight 12- or 16-week phase 2b/3 RCTs have been conducted in North America and Japan, including four positive RCTs but also four RCTs with negative findings. RCT. A recent meta-analysis has analyzed the effect on Off-time reduction relative to placebo, considering all eight RCTs (2719 patients), finding a reduction of − 0.38 h (95% CI − 0.61, − 0.15; p = 0.0011) for 20 mg and − 0.45 h (95% CI − 0.68, − 0.22; p < 0.0001) for 40 mg [24]. Conversely, considering only the four positive RCTs that have been used to granted istradefylline marketing in US, Off-time reduction was − 0.75 h (95% CI − 1.10, − 0.40) for 20 mg/day and − 0.82 h (− 1.17, − 0.47) for 40 mg/day [24]. Based on a global effect of about 40 min/day, and on four negative RCTs, EMA confirmed its recommendation to refuse marketing authorization.
Safety Data
The available evidence suggests that there are minimal differences between formulations of DAAs in the risk of dyskinesia, hallucinations, and AE-related discontinuation, and that available DAAs, except for apomorphine, share the same efficacy profile. The AEs of non-ergot DAAs include nausea, daytime somnolence, confusion, hallucinations, leg oedema, impulse controls disorders (ICDs) and OH [56,57,58]. The most reported ICDs are pathological gambling, hypersexuality, compulsive shopping, and compulsive eating, with a prevalence ranging from 9% to about 18%, being higher among young male patients with longer exposure duration, a pre-PD history of an ICD, personal or family history of substance abuse, and bipolar disorder [58]. Treatment with DAAs is the main risk factor for ICDs, even if they may also occur with LD, MAO-B Is, and amantadine but to a much lower extent [58]. Another safety issue with DAA treatment is excessive daytime sleepiness and sudden sleep attacks [58], whose risk may increment by about threefold among DAAs users compared with all other PD medication users, with no specific difference in sleep attack prevalence among different DAAs [59].
Overall, discontinuation due to AEs occurrence may be an issue, especially if high dose is reached, and particularly for young patients with ICDs or elderly patients with PD with initial cognitive decline. In the PD MED study, which included a relatively old population (mean age = 71 years), 179 (28%) of 632 patients allocated to receive DAAs discontinued treatment because of AEs compared with 11 (2%) of 528 patients on LD (p < 0.0001) [60].
Lowering the dose of the causative DAA is the first step in the management of the treatment-related (TE) AE, but sometimes a complete drug withdrawal is required. In that case, clinicians should be aware about the rare occurrence of DAA withdrawal syndrome, whose risk is low but may increase in advanced stages [61]. Managing DAA withdrawal syndrome often requires restarting the DAA, but at a lower dose, and then down-titrating again at a much slower pace.
MAO-B Is are usually well-tolerated drugs. Uncommon AEs include insomnia, nausea, dizziness, and OH. Drug interactions leading to a serotonin syndrome type may occur on very rare occasions when MAO-B Is are combined with serotonergic and other monoaminergic-acting drugs, such as selective serotonin-reuptake inhibitors, serotonin–norepinephrine reuptake inhibitors and tricyclic drugs. However, the occurrence is extremely rare, ranging from 0 to 0.2% as reported in a large cohort Phase IV study or a survey study, respectively [62, 63]. Finally, MAO-B Is are likely to be less tolerated among elderly patients with PD as highlighted by the PD-MED trail (mean age: 71 years). Indeed, the PD-MED trail showed 7-years probabilities for treatment discontinuation of 72% for MAO-B I, 50% for DA, and 7% for LD (p < 0.0001) [60].
Different COMT-Is, conversely from what described for DAAs and MAO-B Is, do not share the same safety profile [42]. Indeed, safety concerns and regular hepatic enzyme surveillance related to the potential hepatotoxicity of TOL have limited its use in clinical practice over the past decades, due to four cases of liver failure described in 1998 [43]. Beyond those four cases were found in the literature [43]. For this reason, TOL is a second-line treatment for end-of-dose MF. Additionally, in the 2010 Cochrane Review, the intraclass comparison considering ENT and TOL for any AE versus placebo found a higher odds for TOL compared to ENT (ENT: OR 1.85, CI 1.47–2.33; p < 0.00001 vs. TOL: OR 2.89, CI 1.74–4.79; p < 0.0001) [48].
Since ENT increases the bioavailability of LD, dopaminergic AEs are the most frequent, dyskinesia being the most common, at times requiring an extension of the dose interval or a reduction in the dose of LD. The second most-common dopaminergic AE is nausea, followed by insomnia and hallucinations, whose frequency is higher among elderly patients (> 70 years) [45]. Some of the most frequent non-dopaminergic AEs are abdominal pain, diarrhea, and harmless urine discoloration. The latter may appear within weeks or months after starting ENT and may disappear during therapy [42]. OPC share the same safety profile as ENT, with dyskinesia being the most common AE, but with much lower cases of diarrhea and no urine discoloration [64]. For both OPC and ENT, there are no hepatic concerns.
News. In the last 2 years, a few new add-on compounds for the treatment of MF have been evaluated in Phase 2 and Phase 3 trials. Early in 2022, a Phase III randomized double-blind trial has shown the efficacy of a new drug, P2B001, which combines a low dose of pramipexole with a low dose of rasagiline, in a population of de novo patients with PD [65]. P2B001 has shown to have a similar efficacy on the UPDRS-III (no data on Off-time/day), compared to marketed titrated dose of pramipexole ER but a better safety profile, with lower occurrence of sleepiness and OH [65]. Nevertheless, it has never been used in advanced patients with PD with MF, but only in an early disease stage.
Another new DAA, Tavapadon, which is specifically a selective partial agonist of dopamine type 1 (D1 and D5) receptors administered orally once daily is under evaluation in a Phase III at the dose of 5–15 mg/day, for patients with MF (TEMPO 3 study, NCT04542499). The results are still pending.
A negative Phase II trial has recently halted the development of Foliglurax, a positive allosteric modulator of the mGlu4R, for the treatment of patients with PD with MF [66].
Finally, we are expecting for the results of two Phase II trials, one recently concluded on Bumetanide, a sodium/potassium/chloride cotransporter isoform 1 antagonist (CUREPARK, NCT03899324) and one on CVN424 (NCT04191577), a selective and novel GPR6 inverse agonist.
On-demand Therapies
Overview
Despite the above-mentioned available add-on therapies, MF continue to be a clinical need, with many patients presenting residual Off-time, even when the available best medical treatment is applied. Indeed, add-on treatment may provide a reduction in daily Off-time that ranges from 0.5 to 2 h/day versus placebo. Considering that placebo usually reduce Off-time of about 1 h/day, in a patient population with a baseline of about 5–6 h/day of Off-time [48], we arrive at an overall reduction of about 50% of the total Off-time, thus making their introduction not sufficient for good control of motor symptoms. The persistence of troublesome or unpredictable MF is an indication for a device-aided treatment initiation to be considered (see next paragraph), but only a subset of patients with PD accept or are good candidates for second-line treatments [67]. Oral LD typically takes about 60 min to show a benefit, this delay being too long when a patient is experiencing an acute disabling Off episode, with an effect often unreliable, due to possible delayed or partial-On. Dispersible oral formulations of LD/benserazide and LD methylester/carbidopa may reach the duodenum more quickly than standard LD tablets [68, 69]. However, they have not been specifically studied in this indication, with the exception of one negative randomized double-blind trial [70], are available only in some European countries, and their “rapid” effect, which is observed as starting the morning on a fasting stomach, is much less reliable during the day in fed conditions.
Consequently, on-demand rapid therapies have been developed with the aim to rapidly alleviate the Off episode in bypassing the obstacle of the stomach. There are currently three possible on-demand therapies for patients with PD: subcutaneous (SC) apomorphine injection, inhaled LD – CVT 301 (already approved by the FDA and EMA), and sublingual (SL) apomorphine (approved only in US) [71] (Table 2). Intranasal delivery of LD has been also investigated [72], but no efficacy study is still available.
Subcutaneous Apomorphine Injection
Apomorphine is highly lipophilic and is quickly absorbed. Intermitted subcutaneous injection of apomorphine ensures rapid bioavailability, avoiding issues associated with gastrointestinal transit time and first-pass liver metabolism. Despite its efficacy, it is usually underused, as administration of a SC injection can be challenging for patients in the Off condition. The injections are administered by a multidose pen. Usually, the formulation is a solution of apomorphine hydrochloride of 10 or 20 mg/ml. The dose ranges from 2 to 6 mg, up to 5–6 injections/day, starting with the lowest dose, combined with domperidone and increasing with a slow titration [73]. If patient need more injections, it is generally recommended to consider the switch to continuous subcutaneous apomorphine infusion (CSAI) (see “Device-aided therapies” paragraph).
Sublingual Apomorphine
Other routes of administration, except the SC one, have been investigated for apomorphine, including intravenous, intranasal, rectal, sublingual, inhaled and transdermal [74]. None of these has been approved for the market except a SL formulation (APL-1302277) [75]. Of note, in 2013, a small parallel double-blind placebo-controlled group showed the efficacy of inhaled apomorphine 1.5–4.5 mg in rapidly switching the patients from the Off- to On-state in a mean of 5.5 min, although without a significant reduction of total Off-time compared to placebo [76], but no Phase III study was later preformed. SL apomorphine is composed of a soluble bilayer film containing apomorphine in one layer and a pH-controlling buffer that is designed to minimize the risk of skin or mucosal irritation in the other. This formulation is conceived to systemically deliver the drug via absorption from the oral cavity mucosa, thus bypassing the extensive first-pass liver metabolism. Titration can be started at a 5–10 mg dose, which could be increased on subsequent days in 5–10 mg increments to a maximum of 40 mg until a full On-response is achieved [77]. The optimal dose is determined based on individual response, with a titration that can be performed at home without medical supervision, while anti-nausea therapy is not necessary.
Inhaled Levodopa
To overcome the limit of oral LD due to delayed gastric emptying, inhalation powder was developed, allowing the delivering of LD directly into the bloodstream by way of the pulmonary alveoli [78]. This results in a more predictable and faster peak of plasma concentrations following each dose, compared with oral LD. The delivery system consists of dry powder placed into a plastic inhaler, administered in two sequential inhalations, each containing a 42-mg capsule of LD, without carbidopa, as routinely administered with the oral formulation. Up to five daily administrations are allowed. The total LD powder of 84 mg is bioequivalent to 90–100 mg of oral LD.
Efficacy on Off-time Reduction
The efficacy on Off-time reduction of On-demand therapies, after a single dose is typically seen: (1) within 10–15 min of injection and lasting for 45–60 min for SC apomorphine but possible lasting up to 90 min at the dose of 4 mg [79]; (2) within 30 min, lasting 90-min for SL apomorphine [75]; and (3) within 30 min, lasting for 60 min after LD inhalation [80] (Table 2). In an open-label study, SC apomorphine has also been shown to be effective in reducing the time to ON among patients with PD with morning akinesia compared to oral LD (23.72 ± 14.55 min, reduced from 60.86 ± 18.11 min with levodopa; p < 0.0001) [74]. There is no randomized head-to-head RCT comparing one of the On-demand therapies with another, but a recent open-label trial suggested a similar efficacy on motor symptom improvement (based on the MDS-UPDRS part III) at 15 and 90 min for the SC and SL forms of apomorphine, even if patient satisfaction is higher for the SL one [81]. Overall, SC apomorphine is quicker to rapidly switch from the Off- to the On-state compared to SL for inhaled LD, but it can be difficult to manipulate. Regarding inhaled LD, a recent Phase III RCT highlighted that this treatment was effective to improve motor symptoms (UPDRS part III) at 30 min, but not at 20 min, compared to placebo, with higher efficacy for the 84 mg versus 60 mg but with no reduction of total daily Off-time, as assessed by means of a Hauser diary [80]. Of note, in the trial. the drug was not used for the morning akinesia, as it does not contain carbidopa, which could be one of the reasons for its failure on total Off-time reduction, along with the fact that patients used it only twice a day and not up to five times, as allowed [82]. Whether a higher dose of CVT-301 could be well tolerated and more effective remain to be clarified [82].
Safety Data
Overall, for on-demand therapies, local adverse reactions related to the administration route are the most frequent and the principal reason for treatment discontinuation.
SC apomorphine injection is a quite underused rescue therapy, probably due to several constraints, including multiple supervised titration visits, the need to assemble the device and to perform an injection during the Off period, and the classical dopaminergic AEs, including nausea, vomiting, hypotension, and somnolence, summed to local skin reactions [73]. Conversely, no ICDs appearance or increment have been reported. It has been suggested that a lower prevalence of ICD in apomorphine-treated patients compared to orally-treated patients with DAA may be related to the higher affinity of apomorphine for D1 and D2 receptors, in contrast to other DAAs which have a more marked affinity for D3 receptors, but this aspect has been specifically addressed in large cohorts [83].
For SL apomorphine, oropharyngeal mild AEs, including lip/oropharyngeal swelling and oral mucosal erythema, are the most common (up to 31.5%) related to treatment discontinuation in 16.7% of the patients [75]. Other AEs include nausea, somnolence, paraesthesia and dizziness, but no orthostatic hypotension or ICD have been reported. Overall, the frequency of dopaminergic AEs seems to be lower compared to SC administration, probably due to relatively low Cmax.
Finally, inhaled LD has been associated with cough, upper respiratory tract infection, sputum discoloration and nausea, but with no severe pulmonary problems up to 1-year follow-up [84].
News
LC-5, a dispersible micro-tablet formulation for oral intake, containing 5/1.25 mg of LD/carbidopa, has been approved in Sweden since 2014 and in the European Union since 2016. LC-5 allows to fractionate LD in small doses, taken at the onset of MF. The dose is released from the dispenser, preferably into a glass of water where the microtablets are immediately dispersed. The dispenser is equipped with an alarm to facilitate treatment adherence and an optional diary-like function for self-reporting, allowing the individualization and fine tuning of the dose size and interval, as well as a more stable LD plasma concentration compared to standard LD formulation [85]. Of note, in Sweden, LC-5 is reimbursed only for patients with PD who would otherwise be treated with apomorphine or LD infusions.
Surgical and Device-Aided Therapies
Once MF cannot be adequately managed with oral medication, advanced therapies such as surgical and device-aided therapies (DAT) should be considered [86, 87]. Surgical therapies can be divided into non-lesional [deep brain stimulation (DBS) on the subthalamic (STN) and globus pallidum internum (GPi) nucleus] and lesional ones [radiofrequency unilateral pallidotomy and magnetic resonance imaging–guided focused ultrasound (MRgFUS)]. Approved DATs for patients with PD with MF include LCIG and CSAI, while foslevodopa/foscarbidopa continuous subcutaneous infusion (FCSI) is still waiting its formal marketed approval (see “News” section for DAT therapies). All these therapeutic strategies aim to reduce dopaminergic sensitivity by delivering continuous (instead of a pulsatile) stimulation of the dopaminergic terminals.
Overall, all advanced therapies can be proposed for patients with troublesome MC, who respond well to LD, without dementia, with a threshold limit age established at 70, rarely 75 years, only for DBS.
Until now, no RCT comparing the available advanced therapies has been performed, and the question of which advanced therapy should be chosen is one that, for now, has no definitive or well-supported answer [86, 87]. Indeed, decision-making regarding the choice of the treatment to adopt should follow an individualized approach considering patient clinical features and preference and the device-specific applicability, as well as economic costs, depending on the country/health care system. It has been estimated that around 66% of advanced patients with PD may be eligible for at least one advanced therapy [67]. Among them, probably a smaller percentage would be eligible to only one of them due to absolute contraindications, and a higher percentage eligible for more than one of the available therapies. At the end, the choice should be shared between the treating physician, the patient, and the caregiver/family, after a careful and informed discussion about the risks and benefits of each treatment option. Efficacy on MF and recommendations of the MDS and European Academy of Neurology (EAN) for DATs and surgical indications are summarized in Tables 3 and 4, respectively.
Of note, despite their great efficacy in improving motor symptoms, MF, and QoL, the effect of advanced therapies remains symptomatic, and no disease-modifying effect has been proved. However, some authors have recently suggested that long-term controlled studies seem to suggest that STN-DBS may delay some of the late-stage disability milestones and slightly prolong survival compared with matched medically-treated patients [88].
Surgical Non-lesional Therapies
Deep Brain Stimulation
Overview
Introduced by Benabid and colleagues more than 30 years ago, DBS revolutionized the chirurgical treatment for PD. Due to the improvement in tremor upon lesioning of the thalamic ventralis intermedius (Vim), this nucleus was chosen as the first target for neuromodulation in patients with PD. However, and despite the dramatic benefit in tremor, Vim-DBS had no effect on other parkinsonian motor symptoms. After the discovery of the pathophysiologic role of the STN in PD [89], DBS electrodes were implanted in this nucleus. Later, GPi was also introduced as a target for STN-DBS, upon observations of motor improvement after unilateral pallidotomy [90].
In DBS surgery, electrodes are implanted into the deep structures of the brain in order to electrically stimulate specific structures, thereby modulating the dysregulated neural circuits [91]. DBS enables a continuous stimulation, where three main parameters can be adjusted in order to obtain the maximal benefit with the least AEs (larger therapeutic window) [92]. Therapeutic amplitudes for DBS (across all targets) normally range between 1 and 3.6 V. Above that, minimal clinical improvements are observed and there is a significant increase of energetic cost. Frequency is normally set at 130 Hz. Except for tremor, little additional benefit is seen above that, and no additional benefit is seen on frequencies above 200 Hz [92]. Lower frequencies (< 10 Hz) may aggravate parkinsonian signs, but stimulations above 50 Hz can improve some axial symptoms. Pulse width seems to have the least important role in improving clinical signs in DBS patients. It is recommended to start stimulating with the lowest possible pulse width of 60 μs [92].
Recent technological developments with new electrodes enabling directional stimulation will allow innovations in stimulation algorithms and new programming strategies that will further improve the management of patients with DBS-PD [93].
The criteria for the main RCT having evaluated the effect of DBS on Off-time were based on the presence of diagnosis of PD (for more than 5 years), the presence of troublesome MC not controlled by optimal oral medication, the presence of improvement of motor symptoms upon administration of LD and the absence of moderate–severe cognitive impairment or severe and active psychiatric dysfunction [94,95,96]. Patients older than 70–75 years were excluded. These cohorts of patients typically present long disease duration at the time of surgery of 11–14 years, with severe disease-associated motor disability.
Efficacy on Off-time Reduction
The benefit of STN-DBS versus best-medical treatment (BMT) has already been proved in several RCTs [94, 95, 97,98,99], with one additional study comparing both STN- and GPI-stimulated patients with a BMT group [96]. In all of them, stimulation-treated-patients presented larger improvements in the “worst mobility” scores and QoL than the BMT group. Just three of these studies reported the benefits of DBS on Off-time reduction compared to the BMT group [94, 95, 99]. Of note, only the STN target allows a tapering of about 50% of oral dopaminergic medication, which also helps in the management of dyskinesia, while the GPi target does not allow the lowering of oral medications.
In the cohort of Deuschl and colleagues, DBS patients presented a significant Off-time reduction versus BMT of 4.2 h/day versus 0.0 h/day (p < 0.001) [94], while in Weaver’s study, DBS reduced the Off-time by 2.5 h/day (95% CI 1.7–3.2, p < 0.001) when compared to the BMT group [95]. In the EarlySTIM trial, a 1.8-h/day reduction in the Off-time was obtained in the DBS-STN group when compared to BMT (95% CI 0.5–3.1, p = 0.006) [99]. No RCT comparing DBS-GPI to BMT is available, but two RCTs comparing DBS-GPI with DBS-STN found no between-group differences regarding Off-time reduction [96, 100].
Safety
AEs are commonly separated into those related to the surgical procedure (intracranial haemorrhage, postoperative seizures), those that are hardware-related (infections, fracture, dislocation lead, implanted pulse generator malfunction) and those associated with the stimulation itself or with the modification of oral treatment. The prevalence of AEs have been evaluated in several reviews and multicentric retrospective studies, adding to the data included in the RCT. AEs occur in less than half of patients, but the coexistence of more than one AE per patient is not uncommon [101, 102]. Intracerebral hemorrhage is one of the most feared complications. The risk of a symptomatic hemorrhage has been reported to be lower than 1% [103], with a recent review reporting an average rate of 3.4% of intracranial complications including intracerebral hemorrhage, cerebral edema and cerebral abscess [102]. Infection of the hardware is a severe hardware-related complication with an incidence ranging for 1.5–22%. There is no consensus about the best way to manage hardware infections that ultimately can result in hardware removal. An annual average rate of 2.4% of hardware removal and a 2.6% rate of lead revision have been reported [103].
Weight gain may also occur in DBS-treated patients, with PD likely due to an interplay between decrease energy consumption (improved dyskinesia) and stimulation-induced effects on the hypothalamic [104]. Possible mood/behavioral disorder may occur soon after the implantation. The occurrence of apathy after surgery is thought to be related to the reduction of dopaminergic medication, especially with reduction and withdrawal of DAAs, considering their positive effect on apathy [105]. Affective disorders such as depression and (hipo) mania have also been described, and an increased risk of suicide has also been found in patients after DBS surgery, with higher risks for young or single patients with postoperative depression and a previous history of ICDs or compulsive medication use [106,107,108]. To avoid those AEs, a close surveillance of mood modifications and a stepped increment of stimulation voltage, without aggressive medication reduction, is highly recommended in the next month after implantation [109]. Of note, ICD can both improve after surgery or be induced by the stimulation [110]. Other possible AE stimulation-related are dysarthria, gait disorders, dyskinesia, dystonia and decreased verbal fluency [111].
Surgical Lesional Therapies
Overview
Radiofrequency pallidotomy is an ablative procedure where a localized unilateral lesion on GPi can be performed using thermocoagulation. Unilateral posteroventral pallidotomy has been historically replaced by DBS, as it allows only a unilateral procedure due to safety concerns for bilateral intervention, with no effect on axial symptoms and no possibilities to taper antiparkinsonian medications, but it is well effective on MC, with lower risk of infections and surely remain a less expensive attractive approach, for developing countries, or for patients with no possibilities of closed follow-up visits for stimulation setting [112, 113].
Magnetic Resonance Imaging–Guided Focused Ultrasound (MRgFUS) Subthalamotomy is a lesional procedure, that integrates ultrasonic waves with magnetic resonance imaging for therapeutic transcranial ablation. Being an image guided procedure, no incision is performed during the procedure [114]. (40) Its non-invasiveness is a major advantage as well as the fact that the patients don’t need multiple sessions for programming [115].
Efficacy on Off-time Reduction
Two RCTs have compared unilateral pallidotomy with BMT in patients with PD [112, 113], with just one assessing the effect on Off-time reduction [112]. A non-significant Off-time reduction of − 1.4 h/day (95% CI − 3.3 to 0.6, p = 0.15) was observed on the pallidotomy-treated group compared with the BMT group [112]. The efficacy of unilateral subthalamotomy using MRgFUs has been studied in one randomized controlled study [114]. Patients were recruited if they had a highly asymmetric parkinsonism, while the presence of severe dyskinesia was an exclusion criteria. Mild scores on the UPDRS-part IV were observed in these cohort. The amount of Off-time reduction was not assessed, but MF, evaluated using the MDS-UPDRS part IV score, had a reduction of 1.5 points (CI 95% 0.2, 2.7) in the MRgFUS patients compared to the BMT group [63].
Safety
In radio-frequency pallidotomy, transient AEs such as peri-operative confusion and delirium, transitory hemiparesis contralateral to the intervention site and focal seizures may occur rarely [112]. AEs persisting for more than 3 months after surgery have also been reported, with increased dysarthria, dysphagia and sialorrhea being the most commonly reported [112, 113]. Intracerebral hemorrhage has also been reported in a limited number of patients.
Regarding MRgFUS subthalamotomy, 22% of the patients presented Off-medication state dyskinesia [114]. Dyskinesia appeared during the first week after the procedure and, despite progressive improvement in the majority of patients, dyskinesias persistent up to 1 year in some. Intraprocedural adverse events such as headache, dizziness and nausea were common, but resolved completely after the procedure [114]. Hemiparesis contralateral at the treated site was present in 19% of the patients, with the majority progressively recovering completely or almost completely. Dysarthria (56%), gait disturbances (48%) and hemiparesis contralateral to the treated site (19%) appeared shortly after the procedure, with the majority resolving progressively during the follow-up [114].
Device-Aided Therapies
Overview
Continuous Subcutaneous Apomorphine Infusion. CSAI is provided in 10-mL pre-filled glass syringes and delivered as a 5-mg/mL solution for infusion through an infusion pump. Perfusion doses of 3–8 mg/h are normally used, with patients staying on the infusion only during the waking hours, to a total maximum of 16–18 h/day [116]. To achieve the optimal dose for each individual patient, apomorphine is normally started at a 0.5–1 to 1 mg/h and slowly titrated daily at 0.5–1.0 mg/h [116], with the possibility to use extra-bolus on-demand over the day. Exceptionally, perfusion during the whole 24 h can be performed [116]. Several factors can influence its subcutaneous absorption and consequently its efficacy: injection site, state of the skin, volume and depth of injection and the presence of subcutaneous nodules [116]. A recent study highlighted its possible role in improving sleep disturbance when used only as a night-time perfusion among patients with PD with motor complications and insomnia [117].
Levodopa/carbidopa intestinal gel. LCIG is a methylcellulose gel suspension of levodopa/carbidopa specifically created for continuous enteral infusion [118]. The delivery system is comprises a jejunal or duodenal tube placed by a percutaneous endoscopic gastrostomy procedure, and connected to a portable pump and medication cassette containing the gel which is worn attached to the waist or over the shoulder [118]. This continuous mode of administration of levodopa limits the pulsatile stimulation associated with oral administration and reduces variability in levodopa plasma levels [119]. A 16-h levodopa perfusion is normally recommended, although longer times can be used if needed. In addition to the continuous perfusion, a morning-bolus is also given, and additional boluses (on-demand) can also be given throughout the perfusion time [120].
More recently, thanks to the observation that the addition of oral ENT or TOL (every 5 h) to LCIG treatment allowed a decrement of approximately 20% in the LCIG dose, up to 35% of the continuous dose, a new combination of levodopa/ENT/carbidopa intestinal gel (LECIG), has been proposed [121, 122]. However, LECIG is currently approved only in Sweden and Germany. More recently, an observational study, with half of the patients switched directly from LCIG, suggested that this new pump is probably more user-friendly owing to its lower weight and size [123].
Efficacy on Off-time Reduction
A double-blind RCT has evaluated the efficacy of CSAI in advanced patients with PD with MF [124]. In the CSAI-treated group, Off-time was reduced of − 2.47 h/day (± 3.7) representing a reduction of − 1.89 h/day when compared to the BMT group [124]. The benefit was maintained up to a 1-year follow-up, as highlighted by the open-label phase of the trial, showing a mean (SD) change from double-blind baseline in daily Off-time of − 3.66 (2.72) h/day [125].
Regarding LCIG, two RCTs have shown that LCIG treatment provided a significantly greater reduction in Off-time when compared to the BMT group [31, 126]. The first double-blind double-dummy trial (primary end-point: Off-time reduction) showed − 4.04 h/day (SE 0.65) in the LCIG group versus baseline after 12 weeks (with − 1.9 h/day versus oral levodopa). The second open-label randomized trial (Off-time: secondary outcome) showed a reduction of − 2.35 h/day in Off-time compared to BMT (p = 0.0002) [126].
Safety
Although DATs are generally safe, there is a high rate of AEs associated with the device mechanisms which can be in partly minimized using preventive measures and educating patients about the correct use and management of the devices.
CSAI is generally associated with a higher rate of AEs than on-demand apomorphine injections. Cutaneous and subcutaneous AEs related to the administration mode are the most common, including bruising, subcutaneous nodules and, rarely, necrosis or abscess formation at the infusion sites. This cutaneous reaction, although normally not severe, can lead to treatment discontinuing, along with dyskinesia persistence and cognitive impairment [127]. Ensuring a good skin hygiene using new needles for each injection, rotating the injection sites, injection and massaging, and using ultrasound therapy may reduce the risk of skin complications. Nausea, somnolence, hypotension and psychosis are also commonly reported AEs [125, 128], while autoimmune hemolytic anemia and QT interval prolongation despite being rare, due their severity, require ECG and blood count monitorization at baseline and after starting the treatment. Despite being a DA, a low rate of development of ICD was observed in CSAI treated patients [129].
The rate of AE is also high in patients under LCIG, with a high drop-out rate associated of 10% per year [130, 131]. The most common AEs in LCIG patients are device-related: tube dislocations, tube occlusion and accidental tube removal, stoma infection, stoma erythema and granuloma, with all of them potential leading to additional endoscopic procedures. Peritoninis and pneumoperitonitis are rare but life-threatening complications [130, 131]. The most common non–device-related AEs are hallucinations, abdominal pain, and weight loss [132]. Worsening of biphasic dyskinesia may also occur [133, 134], while aggravation of pre-existent chronic polyneuropathy or, more rarely, new onset acute polyneuropathy can happen requiring treatment discontinuation [135, 136].
News
Continuous subcutaneous levodopa infusion (CSLI), including ND0612 and foslevodopa/foscarbidopa (ABBV-951) [137] are new soluble formulations of LD/carbidopa that can be delivered by continuous SC infusion. Their efficacy on MF have been evaluated in a few phases 2/3 trials, but their marketing has been yet not granted. For both, a portable pump is connected to the infusion delivery system and through a cannula inserted subcutaneously in the patient’s abdomen, and the drug is perfused during 24 h. However, ND0612 allows the delivery of about 700 mg/day of LD which implies the concomitant need of oral treatment intake, while ABBV-951 allows higher LD doses, ranging from 600 to 4250 mg/day, even if specific attention to local AEs should be made with high doses.
Data on the effect of ND0612 on MF are available from one Phase 2 RCT and one open-label study, including patients on 24 h/day or 14 h/day infusions. The first, performed on 29 patients, found a reduction in Off-time of − 2.1 ± 2.2 h/day for ND0612 + oral LD and − 1.4 ± 2.3 h/day for placebo + oral LD, after 15 days [138]. The open-label study found an overall reduction in Off-time − 2.0 h/day [CI 95% − 3.3, − 0.7] after 28 days, but this reduction was larger in the 24-h group (− 2.8, CI 95% − 4.6, − 0.9) than in the 14-h group (− 1.3, CI 95% − 3.1, 0.5) [139]. Results of a Phase III trial on ND0612 (BouNDless study, ND0612-317) are expected in early 2023.
For foslevodopa-foscarbidopa (ABBV-951), data regarding the effect on Off-time reduction pertain to one recent Phase III double-blind active control RCT. In this study, the superiority of foslevodopa/foscarbidopa CSCI in reducing the hours spent in “Off-time” was demonstrated, with a 1.79-h/day (95% CI − 3.03, − 0.54, p = 0.0054) reduction compared to the BMT group [140]. Finally, a new oral continuous delivery of LD (dent device) have been recently evaluated in a Phase II trial (NCT04778176) on 18 patients with PD with MF, alternatively submitted to continuous and standard intermittent LD administration over 8 h [141]. Continuous treatment consisted in sips of an LD dispersion at 5- to 10-min intervals. Continuous administration allowed a reduction in Off-time normalized to a 16-h waking day of approximately 1.9 h (p < 0.001) [141].
Regarding the safety profile, infusion site-related side effects are the most common AE. Skin erythema, pain, cellulitis and oedema have been reported, and can be associated treatment discontinuation. Hallucinations and psychosis were also reported as occurring in the foslevodopa/foscabidopa-treated patients, especially those with concomitant DAAs.
Expert Opinion and Conclusions
MF are one of the major sources of disability for patients with PD, and mild MF may start in early disease stages. Neurologists can use a wide armamentarium of oral, surgical and DATs to manage these symptoms.
Regarding oral strategies, there is no consensus on which one should be used first, or if one should be preferred over another. The choice of the right oral treatment for MF is mainly based on the patient’s age, ease of use, cognitive status, on-going treatments, and availability, with mild differences in terms of efficacy that need to be considered (Fig. 2), ranging from − 1.5 to − 0.8 h/day of Off-reduction, compared to placebo. [142]. Of note, it should be considered that a threshold of a minimal clinical important difference for Off-time ranges from 1 to 1.3 h/day [143, 144] has been suggested, possibly indicating that a reduction > 1 h/day is probably more perceived by patients. Overall, DAAs are a convenient once-a-day add-on therapy, slightly more potent compared to MAO-B Is or COMT-Is, but their use can be limited by AE frequency, in particular by daytime somnolence and ICDs. Compared to DAAs, MAO-B Is are a less-potent add-on therapy, but generally well tolerated and equally convenient due to a once-a-day administration. The same is true for istradefylline, which has a mild effect on Off-time reduction, but should possibly be considered in the case of patients at risk for dopaminergic AEs, as it has a non-dopaminergic mechanism of action. Among COMT-Is, OPC has recently gained a relevant role, thanks to its efficacy and its convenient once-a-day administration. It is in discussion if it could be considered one of the first-line treatments for early MF, considering its ability to increase LD bioavailability with avoidance of trough levels, even when reducing its daily dose. To better address this point, a Phase 4 randomized trial is on-going, comparing OPC 50 mg versus LD 100 mg as a first strategy for the treatment of wearing-off (ADOPTION study, NCT04990284).
Algorithm for treatment options for the management of MF, from the early advanced stage to the appearance of troublesome MF in the severe advanced stage. Dotted lines indicate treatments with lower levels of evidence (to be considered with limitations, i.e. radiofrequency pallidotomy) or with few data available and still not marketed (CLSI)
At the same time, clinicians should be aware that most oral add-on therapies are associated with an increment of dyskinesia. This is the reason why the recently proposed role of amantadine ER (ADS5012) in the treatment of Off-time has gained a particularly interesting role that could potentially change our routine PD management, as it could be the only add-on therapy concomitantly able to treat Off-time and dyskinesia, thanks to its double dopaminergic and anti-glutamatergic effect. This aspect merits further investigation in studies specifically powered for Off-time reduction.
Regarding global tolerability, considerations should be made considering patient age and cognitive status, frequently narrowing the therapeutic possibilities. DAAs should be avoided among elderly patients (> 70–75 years) or used at low doses, especially in cases of cognitive impairment. The same is true for all other oral add-on therapies, including COMT-Is and MAO-B Is, as LD has a better safety profile among those fragile patients with PD. At the same time, we should consider that late-stage patients with PD usually have a lower frequency of troublesome MF, despite severe axial and cognitive disturbances, compared to earlier stages, though not requiring a complex oral management, as they are frequently treated with LD monotherapy. New and interesting compounds in terms of tolerability should be available in the next few years: (1) P2B001, that has shown to be associated with a lower incidence of somnolence, and OH with equal motor benefit in de novo patients with PD, suggesting that it could be worth verifying its efficacy on MF and its long-term safety profile, and its tolerability among elderly patients in larger observational trials, and (2) Tavapadon, as D1 receptor agonists may have a better tolerability compared to the common D2-D3 DAAs.
Of note, even if the armamentarium of advanced therapies is widening, with a global tendency to propose them more and earlier in the course of the disease, most patients with PD remain on oral treatment, for their entire life course. In this sense, finding the right combination of oral treatments for MF remains a required ability for physicians treating patients with PD.
Entering the domain of advanced therapies, their initiation should be a shared decision, ideally made by a team of healthcare professionals, experienced in all the available therapies, along with patients and caregivers.
Looking at the three “classical” advanced therapies, i.e. CSAI, DBS and LCIG, the choice involves a complex decision process that weights not only the severity of Off-time but also the concomitant presence of other troublesome symptoms, such as dyskinesia or NMS. The presence of contraindications that may compromise eligibility for one specific treatment option, and the patients’ own preference should also be considered during the decision-making process. As caregivers are usually required for all the DATs, except DBS, involving them in the discussion of a shared decision is also of paramount importance. The recently published EAN guidelines for advanced therapies indicated STN-DBS as the one with the highest level of effect in terms of daily Off-time reduction compared to other advanced therapies [145], as well as having a considerable effect on early MF, as suggested by the EARLY-STIM trial. On this path, DBS has been approved by the FDA for patients with PD with at least 4 years of disease duration and 4 months of MC, who are not adequately controlled by medications. However, the right time for DBS is still a matter of discussion, and early indications are still not universally accepted. Indeed, the rate of severe AEs, including surgical, hardware-related, and behavioural ones, is probably underestimated in the literature, as mostly based on RCTs data and not real-life cohorts/database [146, 147]. Thus, surgical indication, especially for young patients, who can be adequately controlled by oral medications, could wait and be considered if patients’ QoL and autonomy are frankly impaired with non-invasive treatment, as a high degree of disability and a severe QoL impairment are good prognostic factors for positive DBS outcomes.
On a related note, no RCT has investigated the effect of LCIG on early fluctuations, while a randomized open-label Phase IIII trial is on-going to investigate QoL (as primary out-come) improvement due to CSAI in early fluctuators (at least 4 years of disease duration and 3 years of MC; NCT02864004, EARLY-PUMP study).
Among advanced therapies, the most recent new treatment options are CSLI and MRgFUS, even if only the latter has so far been approved, although still only available in a few countries. At the same time, as highlighted by the recent EAN guideline for invasive therapies, MRgFUS is still not recommended for the management of MF, due to the absence of data related to Off-time reduction. Likewise, CSLI, though promising and with proved efficacy shown in a Phase III active controlled RCT, would need longer follow-up studies focusing on tolerability and drop-out rate, considering that the rate of drop-out was about 30% in the active arm, over 12 weeks of treatment. Additionally, CSLI only allows a limited levodopa dose infused/day, and it the best profile of patients with PD more suitable for this treatment option has yet to be clarified, i.e. whether more indicated for early MF to smooth pulsatile levodopa pick or for all advanced-stage patients.
Among the spectra of MF, unpredictable/sudden Off-periods and NMF still remain a neglected phenotype in terms of treatment options. Indeed, no RCT with an oral compound has indicated, as primary outcome, the improvement of severe/unpredictable MF (and dyskinesia), and patients with this kind of severe MC are sometimes also excluded a priori. The advent of different rescue therapies has certainly improved the management of sudden Off-periods, even if they are quite recent and still probably underused. Advanced therapies are the only ones indicated for severe MF, including as first option, DBS and LCIG, and as a second option, CSAI, even if related trials have included all kinds of MF and not just the unpredictable ones [22, 145]. Despite this effect on several NMS being suggested for both DBS, CSAI and LCIG at short/intermediate follow-up [148], there is actually no study specifically focused on NMF. This point remains to be investigated.
Finally, clinicians should keep in mind that oral, surgical and DATs are not unique add-on treatments for patients with PD. Over the last decade, the place of exercise has gained more and more importance in the improvement of parkinsonian motor symptoms, even with the main large randomized trials targeting early patients with PD. Indeed, a positive effect of aerobic exercise at a defined frequency/week has been shown on PD motor symptoms [149,150,151]. However, its role in the reduction of Off-time has never been specifically addressed, nor its effect on dopaminergic drug absorption.
Change history
02 February 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40120-023-00441-4
References
Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–6.
Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, et al. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018;33(8):1248–66.
Tambasco N, Romoli M, Calabresi P. Levodopa in Parkinson’s disease: current status and future developments. Curr Neuropharmacol. 2018;16(8):1239–52.
Chapuis S, Ouchchane L, Metz O, Gerbaud L, Durif F. Impact of the motor complications of Parkinson’s disease on the quality of life. Mov Disord. 2005;20(2):224–30.
Warren Olanow C, Kieburtz K, Rascol O, Poewe W, Schapira AH, Emre M, et al. Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov Disord. 2013;28(8):1064–71.
Politis M, Wu K, Molloy S, Bain PG, Chaudhuri KR, Piccini P. Parkinson’s disease symptoms: the patient’s perspective. Mov Disord. 2010;25(11):1646–51.
Lieberman AVM. Wearing-off of levodopa of greatest concern for PD patients. Eur J Neurol. 2014;Suppl 2:190.
Cilia R, Akpalu A, Sarfo FS, Cham M, Amboni M, Cereda E, et al. The modern pre-levodopa era of Parkinson’s disease: insights into motor complications from sub-Saharan Africa. Brain. 2014;137(Pt 10):2731–42.
LeWitt PA. Levodopa therapy for Parkinson’s disease: pharmacokinetics and pharmacodynamics. Mov Disord. 2015;30(1):64–72.
Schrag A, Quinn N. Dyskinesias and motor fluctuations in Parkinson’s disease. A community-based study. Brain. 2000;123(Pt 11):2297–305.
Nutt JG. Motor fluctuations and dyskinesia in Parkinson’s disease. Parkinsonism Relat Disord. 2001;8(2):101–8.
Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, et al. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351(24):2498–508.
Aquino CC, Fox SH. Clinical spectrum of levodopa-induced complications. Mov Disord. 2015;30(1):80–9.
Bhidayasiri R, Trenkwalder C. Getting a good night sleep? The importance of recognizing and treating nocturnal hypokinesia in Parkinson’s disease. Parkinsonism Relat Disord. 2018;50:10–8.
Hauser RA, McDermott MP, Messing S. Factors associated with the development of motor fluctuations and dyskinesias in Parkinson disease. Arch Neurol. 2006;63(12):1756–60.
Stocchi F, Jenner P, Obeso JA. When do levodopa motor fluctuations first appear in Parkinson’s disease? Eur Neurol. 2010;63(5):257–66.
Stocchi F, Coletti C, Bonassi S, Radicati FG, Vacca L. Early-morning OFF and levodopa dose failures in patients with Parkinson’s disease attending a routine clinical appointment using Time-to-ON Questionnaire. Eur J Neurol. 2019;26(5):821–6.
Franke C, Storch A. Nonmotor fluctuations in Parkinson’s disease. Int Rev Neurobiol. 2017;134:947–71.
Martinez-Fernandez R, Schmitt E, Martinez-Martin P, Krack P. The hidden sister of motor fluctuations in Parkinson’s disease: a review on nonmotor fluctuations. Mov Disord. 2016;31(8):1080–94.
Nutt JG, Holford NH. The response to levodopa in Parkinson’s disease: imposing pharmacological law and order. Ann Neurol. 1996;39(5):561–73.
Chaudhuri KR, Rizos A, Sethi KD. Motor and nonmotor complications in Parkinson’s disease: an argument for continuous drug delivery? J Neural Transm (Vienna). 2013;120(9):1305–20.
Ferreira JJ, Katzenschlager R, Bloem BR, Bonuccelli U, Burn D, Deuschl G, et al. Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson’s disease. Eur J Neurol. 2013;20(1):5–15.
Hauser RA, Lytle J, Formella AE, Tanner CM. Amantadine delayed release/extended release capsules significantly reduce OFF time in Parkinson’s disease. NPJ Parkinson’s Dis. 2022;8(1):29.
Hauser RA, Hattori N, Fernandez H, Isaacson SH, Mochizuki H, Rascol O, et al. Efficacy of istradefylline, an adenosine A2A receptor antagonist, as adjunctive therapy to levodopa in Parkinson’s disease: a pooled analysis of 8 phase 2b/3 trials. J Parkinsons Dis. 2021;11(4):1663–75.
Mizuno Y, Kondo T. Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson’s disease. Mov Disord. 2013;28(8):1138–41.
Freitas ME, Ruiz-Lopez M, Fox SH. Novel levodopa formulations for Parkinson’s disease. CNS Drugs. 2016;30(11):1079–95.
Modi NB, Mittur A, Dinh P, Rubens R, Gupta S. Pharmacodynamics, efficacy, and safety of IPX203 in Parkinson disease patients with motor fluctuations. Clin Neuropharmacol. 2019;42(5):149–56.
LeWitt PA, Giladi N, Navon N. Pharmacokinetics and efficacy of a novel formulation of carbidopa-levodopa (Accordion Pill(®)) in Parkinson’s disease. Parkinsonism Relat Disord. 2019;65:131–8.
Ondo W. IPX066, a mixed immediate/sustained-release levodopa preparation for Parkinson’s disease. Expert Opin Pharmacother. 2014;15(14):2081–5.
Müller T, Möhr JD. Efficacy of carbidopa-levodopa extended-release capsules (IPX066) in the treatment of Parkinson disease. Expert Opin Pharmacother. 2018;19(18):2063–71.
Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13(2):141–9.
Hauser RA, Hsu A, Kell S, Espay AJ, Sethi K, Stacy M, et al. Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson’s disease and motor fluctuations: a phase 3 randomised, double-blind trial. Lancet Neurol. 2013;12(4):346–56.
Stocchi F, Hsu A, Khanna S, Ellenbogen A, Mahler A, Liang G, et al. Comparison of IPX066 with carbidopa-levodopa plus entacapone in advanced PD patients. Parkinsonism Relat Disord. 2014;20(12):1335–40.
Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16(3):448–58.
Hauser RA EA, LeWitt P, Ellenbogen A, Isaacson S, Pahwa R, Stocchi F, Visser H, D’Souza R. A phase 3 trial of IPX203 vs CD-LD IR in Parkinson’s disease patients with motor fluctuations (RISE-PD) May 03, 2022; 98(18 Supplement)
Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G. Valvular heart disease and the use of dopamine agonists for Parkinson’s disease. N Engl J Med. 2007;356(1):39–46.
Fox SH, Katzenschlager R, Lim SY, Ravina B, Seppi K, Coelho M, et al. The movement disorder society evidence-based medicine review update: treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2011;26(Suppl 3):S2-41.
Jenner P, Katzenschlager R. Apomorphine - pharmacological properties and clinical trials in Parkinson’s disease. Parkinsonism Relat Disord. 2016;33(Suppl 1):S13-s21.
Fernandez HH, Chen JJ. Monoamine oxidase-B inhibition in the treatment of Parkinson’s disease. Pharmacotherapy. 2007;27(12 Pt 2):174s-s185.
Fabbri M, Rosa MM, Abreu D, Ferreira JJ. Clinical pharmacology review of safinamide for the treatment of Parkinson’s disease. Neurodegener Dis Manag. 2015;5(6):481–96.
Murata M, Hasegawa K, Kanazawa I, Fukasaka J, Kochi K, Shimazu R. Zonisamide improves wearing-off in Parkinson’s disease: a randomized, double-blind study. Mov Disord. 2015;30(10):1343–50.
Fabbri M, Ferreira JJ, Rascol O. COMT inhibitors in the management of Parkinson’s disease. CNS Drugs. 2022;36(3):261–82.
Artusi CA, Sarro L, Imbalzano G, Fabbri M, Lopiano L. Safety and efficacy of tolcapone in Parkinson’s disease: systematic review. Eur J Clin Pharmacol. 2021;77(6):817–29.
Schrag A. Entacapone in the treatment of Parkinson’s disease. Lancet Neurol. 2005;4(6):366–70.
Fabbri M, Ferreira JJ, Lees A, Stocchi F, Poewe W, Tolosa E, et al. Opicapone for the treatment of Parkinson’s disease: a review of a new licensed medicine. Mov Disord. 2018;33(10):1528–39.
Kondo T, Mizuno Y. A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clin Neuropharmacol. 2015;38(2):41–6.
Rascol O, Fabbri M, Poewe W. Amantadine in the treatment of Parkinson’s disease and other movement disorders. Lancet Neurol. 2021;20(12):1048–56.
Stowe R, Ives N, Clarke CE, Deane K, Wheatley K, Gray R, et al. Evaluation of the efficacy and safety of adjuvant treatment to levodopa therapy in Parkinson s disease patients with motor complications. Cochrane Database Syst Rev. 2010;7(7):CD007166.
Ferreira JJ, Lees A, Rocha JF, Poewe W, Rascol O, Soares-da-Silva P. Opicapone as an adjunct to levodopa in patients with Parkinson’s disease and end-of-dose motor fluctuations: a randomised, double-blind, controlled trial. Lancet Neurol. 2016;15(2):154–65.
Lees AJ, Ferreira J, Rascol O, Poewe W, Rocha JF, McCrory M, et al. Opicapone as adjunct to levodopa therapy in patients with parkinson disease and motor fluctuations: a randomized clinical trial. JAMA Neurol. 2017;74(2):197–206.
LA Ferreira JJ, Rascol O, Poewe O, Santos A, Rocha J, Soares-Da-Silva P. Switch to Opicapone from Entacapone based on experience in BIPARK-I study. Eur J Neurol. 2017;24(Suppl 1):445–678.
Ferreira JJ, Poewe W, Rascol O, Stocchi F, Antonini A, Moreira J, et al. Effect of opicapone on levodopa pharmacokinetics in patients with fluctuating Parkinson’s disease. Mov Disord. 2022;37(11):2272–83.
Borgohain R, Szasz J, Stanzione P, Meshram C, Bhatt M, Chirilineau D, et al. Randomized trial of safinamide add-on to levodopa in Parkinson’s disease with motor fluctuations. Mov Disord. 2014;29(2):229–37.
Borgohain R, Szasz J, Stanzione P, Meshram C, Bhatt MH, Chirilineau D, et al. Two-year, randomized, controlled study of safinamide as add-on to levodopa in mid to late Parkinson’s disease. Mov Disord. 2014;29(10):1273–80.
Cattaneo C, Sardina M, Bonizzoni E. Safinamide as add-on therapy to levodopa in mid- to late-stage Parkinson’s disease fluctuating patients: post hoc analyses of studies 016 and SETTLE. J Parkinsons Dis. 2016;6(1):165–73.
Ceravolo R, Rossi C, Del Prete E, Bonuccelli U. A review of adverse events linked to dopamine agonists in the treatment of Parkinson’s disease. Expert Opin Drug Saf. 2016;15(2):181–98.
Antonini A, Tolosa E, Mizuno Y, Yamamoto M, Poewe WH. A reassessment of risks and benefits of dopamine agonists in Parkinson’s disease. The Lancet Neurology. 2009;8(10):929–37.
Voon V, Napier TC, Frank MJ, Sgambato-Faure V, Grace AA, Rodriguez-Oroz M, et al. Impulse control disorders and levodopa-induced dyskinesias in Parkinson’s disease: an update. Lancet Neurol. 2017;16(3):238–50.
Avorn J, Schneeweiss S, Sudarsky LR, Benner J, Kiyota Y, Levin R, et al. Sudden uncontrollable somnolence and medication use in Parkinson disease. Arch Neurol. 2005;62(8):1242–8.
Gray R, Ives N, Rick C, Patel S, Gray A, Jenkinson C, et al. Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease (PD MED): a large, open-label, pragmatic randomised trial. Lancet (London, England). 2014;384(9949):1196–205.
Cunnington AL, White L, Hood K. Identification of possible risk factors for the development of dopamine agonist withdrawal syndrome in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(9):1051–2.
Panisset M, Chen JJ, Rhyee SH, Conner J, Mathena J. Serotonin toxicity association with concomitant antidepressants and rasagiline treatment: retrospective study (STACCATO). Pharmacotherapy. 2014;34(12):1250–8.
Richard IH, Kurlan R, Tanner C, Factor S, Hubble J, Suchowersky O, et al. Serotonin syndrome and the combined use of deprenyl and an antidepressant in Parkinson’s disease. Parkinson Study Group. Neurology. 1997;48(4):1070–7.
Lees A, Ferreira JJ, Rocha JF, Rascol O, Poewe W, Gama H, et al. Safety Profile of Opicapone in the Management of Parkinson’s Disease. J Parkinsons Dis. 2019;9(4):733–40.
Hauser RA, Giladi N, Poewe W, Brotchie J, Friedman H, Oren S, et al. P2B001 (Extended Release Pramipexole and Rasagiline): a new treatment option in development for Parkinson’s disease. Adv Ther. 2022;39(5):1881–94.
Rascol O, Medori R, Baayen C, Such P, Meulien D. A randomized, double-blind, controlled phase II study of Foliglurax in Parkinson’s disease. Mov Disord. 2022;37(5):1088–93.
Fasano A, Fung VSC, Lopiano L, Elibol B, Smolentseva IG, Seppi K, et al. Characterizing advanced Parkinson’s disease: OBSERVE-PD observational study results of 2615 patients. BMC Neurol. 2019;19(1):50.
Stocchi F, Fabbri L, Vecsei L, Krygowska-Wajs A, Monici Preti PA, Ruggieri SA. Clinical efficacy of a single afternoon dose of effervescent levodopa-carbidopa preparation (CHF 1512) in fluctuating Parkinson disease. Clin Neuropharmacol. 2007;30(1):18–24.
Stocchi F, Vacca L, Grassini P, Pawsey S, Whale H, Marconi S, et al. L-dopa pharmacokinetic profile with effervescent melevodopa/carbidopa versus standard-release levodopa/carbidopa tablets in Parkinson’s disease: a randomised study. Parkinson’s Dis. 2015;2015: 369465.
Stocchi F, Zappia M, Dall’Armi V, Kulisevsky J, Lamberti P, Obeso JA. Melevodopa/carbidopa effervescent formulation in the treatment of motor fluctuations in advanced Parkinson’s disease. Mov Disord. 2010;25(12):1881–7.
Olanow CW, Poewe W, Rascol O, Stocchi F. On-demand therapy for OFF episodes in Parkinson’s disease. Mov Disord. 2021;36(10):2244–53.
Nedorubov AA, Pavlov AN, Pyatigorskaya NV, Brkich GE, Shabalina MM. Pharmacokinetics of nanosomal form of levodopa in intranasal administration. Open access Macedonian J Med Sci. 2019;7(21):3509–13.
Chen JJ, Obering C. A review of intermittent subcutaneous apomorphine injections for the rescue management of motor fluctuations associated with advanced Parkinson’s disease. Clin Ther. 2005;27(11):1710–24.
Koller W, Stacy M. Other formulations and future considerations for apomorphine for subcutaneous injection therapy. Neurology. 2004;62(6 Suppl 4):S22–6.
Olanow CW, Factor SA, Espay AJ, Hauser RA, Shill HA, Isaacson S, et al. Apomorphine sublingual film for off episodes in Parkinson’s disease: a randomised, double-blind, placebo-controlled phase 3 study. Lancet Neurol. 2020;19(2):135–44.
Grosset KA, Malek N, Morgan F, Grosset DG. Inhaled apomorphine in patients with “on-off” fluctuations: a randomized, double-blind, placebo-controlled, clinic and home based, parallel-group study. J Parkinsons Dis. 2013;3(1):31–7.
Hui JS, Fox SH, Neeson W, Bhargava P, Pappert E, Blum D, et al. Open-label titration of apomorphine sublingual film in patients with Parkinson’s disease and “OFF” episodes. Parkinsonism Relat Disord. 2020;79:110–6.
LeWitt PA, Hauser RA, Grosset DG, Stocchi F, Saint-Hilaire MH, Ellenbogen A, et al. A randomized trial of inhaled levodopa (CVT-301) for motor fluctuations in Parkinson’s disease. Mov Disord. 2016;31(9):1356–65.
Pahwa R, Koller WC, Trosch RM, Sherry JH. Subcutaneous apomorphine in patients with advanced Parkinson’s disease: a dose-escalation study with randomized, double-blind, placebo-controlled crossover evaluation of a single dose. J Neurol Sci. 2007;258(1–2):137–43.
LeWitt PA, Hauser RA, Pahwa R, Isaacson SH, Fernandez HH, Lew M, et al. Safety and efficacy of CVT-301 (levodopa inhalation powder) on motor function during off periods in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Neurol. 2019;18(2):145–54.
Stocchi F RO, Poewe W, Chaudhuri R, Kassubek J, Lopez Manzanares L, Leta V, Zhang I, Bowling A, Wu S, Pappert E. Efficacy of apomorphine sublingual film versus subcutaneous apomorphine for the treatment of OFF episodes in Parkinson’s disease. Mov Disord. 2022; 37 (suppl 1).
Rascol O. CVT-301 for Parkinson’s disease: dose and effect size issues. Lancet Neurol. 2019;18(2):128–30.
Samuel M, Rodriguez-Oroz M, Antonini A, Brotchie JM, Ray Chaudhuri K, Brown RG, et al. Management of impulse control disorders in Parkinson’s disease: controversies and future approaches. Mov Disord. 2015;30(2):150–9.
Grosset DG, Dhall R, Gurevich T, Kassubek J, Poewe WH, Rascol O, et al. Inhaled levodopa in Parkinson’s disease patients with OFF periods: a randomized 12-month pulmonary safety study. Parkinsonism Relat Disord. 2020;71:4–10.
Grétarsdóttir HM, Widman E, Johansson A, Nyholm D. Personalized medicine approach in treating Parkinson’s disease, using oral administration of levodopa/carbidopa microtablets in clinical practice. J Personaliz Med. 2021;11(8):720.
Antonini A, Stoessl AJ, Kleinman LS, Skalicky AM, Marshall TS, Sail KR, et al. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: a multi-country Delphi-panel approach. Curr Med Res Opin. 2018;34(12):2063–73.
Dijk JM, Espay AJ, Katzenschlager R, de Bie RMA. The choice between advanced therapies for Parkinson’s disease patients: why, what, and when? J Parkinsons Dis. 2020;10(s1):S65-s73.
Mahlknecht P, Foltynie T, Limousin P, Poewe W. How does deep brain stimulation change the course of Parkinson’s disease? Mov Disord. 2022;37(8):1581–92.
Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science (New York, NY). 1990;249(4975):1436–8.
Siegfried J, Lippitz B. Bilateral chronic electrostimulation of ventroposterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery. 1994;35(6):1126–9 (discussion 9–30).
Harmsen IE, Wolff Fernandes F, Krauss JK, Lozano AM. Where are we with deep brain stimulation? A review of scientific publications and ongoing research. Stereotact Funct Neurosurg. 2022;100(3):184–97.
Volkmann J, Herzog J, Kopper F, Deuschl G. Introduction to the programming of deep brain stimulators. Mov Disord. 2002;17(Suppl 3):S181–7.
Krauss JK, Lipsman N, Aziz T, Boutet A, Brown P, Chang JW, et al. Technology of deep brain stimulation: current status and future directions. Nat Rev Neurol. 2021;17(2):75–87.
Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355(9):896–908.
Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ Jr, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301(1):63–73.
Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2010;362(22):2077–91.
Okun MS, Gallo BV, Mandybur G, Jagid J, Foote KD, Revilla FJ, et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson’s disease: an open-label randomised controlled trial. Lancet Neurol. 2012;11(2):140–9.
Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol. 2010;9(6):581–91.
Schuepbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, et al. Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med. 2013;368(7):610–22.
Odekerken VJ, Boel JA, Schmand BA, de Haan RJ, Figee M, van den Munckhof P, et al. GPi vs STN deep brain stimulation for Parkinson disease: three-year follow-up. Neurology. 2016;86(8):755–61.
Seijo FJ, Alvarez-Vega MA, Gutierrez JC, Fdez-Glez F, Lozano B. Complications in subthalamic nucleus stimulation surgery for treatment of Parkinson’s disease. Review of 272 procedures. Acta Neurochir. 2007;149(9):867–75 (discussion 76).
Engel K, Huckhagel T, Gulberti A, Pötter-Nerger M, Vettorazzi E, Hidding U, et al. Towards unambiguous reporting of complications related to deep brain stimulation surgery: a retrospective single-center analysis and systematic review of the literature. PLoS One. 2018;13(8): e0198529.
Kocabicak E, Temel Y. Deep brain stimulation of the subthalamic nucleus in Parkinson’s disease: surgical technique, tips, tricks and complications. Clin Neurol Neurosurg. 2013;115(11):2318–23.
Balestrino R, Baroncini D, Fichera M, Donofrio CA, Franzin A, Mortini P, et al. Weight gain after subthalamic nucleus deep brain stimulation in Parkinson’s disease is influenced by dyskinesias’ reduction and electrodes’ position. Neurol Sci. 2017;38(12):2123–9.
Zoon TJC, van Rooijen G, Balm G, Bergfeld IO, Daams JG, Krack P, et al. Apathy induced by subthalamic nucleus deep brain stimulation in Parkinson’s disease: a meta-analysis. Mov Disord. 2021;36(2):317–26.
Prange S, Lin Z, Nourredine M, Danaila T, Laurencin C, Lagha-Boukbiza O, et al. Limbic stimulation drives mania in STN-DBS in Parkinson disease: a prospective study. Ann Neurol. 2022;92(3):411–7.
Giannini G, Francois M, Lhommée E, Polosan M, Schmitt E, Fraix V, et al. Suicide and suicide attempts after subthalamic nucleus stimulation in Parkinson disease. Neurology. 2019;93(1):e97–105.
Voon V, Krack P, Lang AE, Lozano AM, Dujardin K, Schüpbach M, et al. A multicentre study on suicide outcomes following subthalamic stimulation for Parkinson’s disease. Brain. 2008;131(Pt 10):2720–8.
Okun MS, Wu SS, Fayad S, Ward H, Bowers D, Rosado C, et al. Acute and Chronic Mood and Apathy Outcomes from a randomized study of unilateral STN and GPi DBS. PLoS One. 2014;9(12): e114140.
Kasemsuk C, Oyama G, Hattori N. Management of impulse control disorders with deep brain stimulation: a double-edged sword. J Neurol Sci. 2017;374:63–8.
Buhmann C, Huckhagel T, Engel K, Gulberti A, Hidding U, Poetter-Nerger M, et al. Adverse events in deep brain stimulation: a retrospective long-term analysis of neurological, psychiatric and other occurrences. PLoS One. 2017;12(7): e0178984.
de Bie RM, de Haan RJ, Nijssen PC, Rutgers AW, Beute GN, Bosch DA, et al. Unilateral pallidotomy in Parkinson’s disease: a randomised, single-blind, multicentre trial. Lancet (London, England). 1999;354(9191):1665–9.
Vitek JL, Bakay RA, Freeman A, Evatt M, Green J, McDonald W, et al. Randomized trial of pallidotomy versus medical therapy for Parkinson’s disease. Ann Neurol. 2003;53(5):558–69.
Martínez-Fernández R, Máñez-Miró JU, Rodríguez-Rojas R, Del Álamo M, Shah BB, Hernández-Fernández F, et al. Randomized trial of focused ultrasound subthalamotomy for Parkinson’s disease. N Engl J Med. 2020;383(26):2501–13.
Máñez-Miró JU, Rodríguez-Rojas R, Del Álamo M, Martínez-Fernández R, Obeso JA. Present and future of subthalamotomy in the management of Parkinson’s disease: a systematic review. Expert Rev Neurother. 2021;21(5):533–45.
Carbone F, Djamshidian A, Seppi K, Poewe W. Apomorphine for Parkinson’s disease: efficacy and safety of current and new formulations. CNS Drugs. 2019;33(9):905–18.
De Cock VC, Dodet P, Leu-Semenescu S, Aerts C, Castelnovo G, Abril B, et al. Safety and efficacy of subcutaneous night-time only apomorphine infusion to treat insomnia in patients with Parkinson’s disease (APOMORPHEE): a multicentre, randomised, controlled, double-blind crossover study. Lancet Neurol. 2022;21(5):428–37.
Wirdefeldt K, Odin P, Nyholm D. Levodopa-carbidopa intestinal gel in patients with Parkinson’s disease: a systematic review. CNS Drugs. 2016;30(5):381–404.
Nyholm D, Odin P, Johansson A, Chatamra K, Locke C, Dutta S, et al. Pharmacokinetics of levodopa, carbidopa, and 3-O-methyldopa following 16-hour jejunal infusion of levodopa-carbidopa intestinal gel in advanced Parkinson’s disease patients. AAPS J. 2013;15(2):316–23.
Amjad F, Bhatti D, Davis TL, Oguh O, Pahwa R, Kukreja P, et al. Current practices for outpatient initiation of levodopa-carbidopa intestinal gel for management of advanced Parkinson’s disease in the United States. Adv Ther. 2019;36(9):2233–46.
Nyholm D, Johansson A, Lennernäs H, Askmark H. Levodopa infusion combined with entacapone or tolcapone in Parkinson disease: a pilot trial. Eur J Neurol. 2012;19(6):820–6.
Senek M, Nielsen EI, Nyholm D. Levodopa-entacapone-carbidopa intestinal gel in Parkinson’s disease: a randomized crossover study. Mov Disord. 2017;32(2):283–6.
Öthman M, Widman E, Nygren I, Nyholm D. Initial experience of the Levodopa-Entacapone-Carbidopa intestinal gel in clinical practice. J Personaliz Med. 2021;11(4):254.
Katzenschlager R, Poewe W, Rascol O, Trenkwalder C, Deuschl G, Chaudhuri KR, Henriksen T, van Laar T, Spivey K, Vel S, Staines H, Lees A. Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2018;17(9):749–59.
Katzenschlager R, Poewe W, Rascol O, Trenkwalder C, Deuschl G, Chaudhuri KR, et al. Long-term safety and efficacy of apomorphine infusion in Parkinson’s disease patients with persistent motor fluctuations: results of the open-label phase of the TOLEDO study. Parkinsonism Relat Disord. 2021;83:79–85.
Freire-Alvarez E, Kurča E, Lopez Manzanares L, Pekkonen E, Spanaki C, Vanni P, et al. Levodopa-carbidopa intestinal gel reduces dyskinesia in Parkinson’s disease in a randomized trial. Mov Disord. 2021;36(11):2615–23.
Olivola E, Fasano A, Varanese S, Lena F, Santilli M, Femiano C, et al. Continuous subcutaneous apomorphine infusion in Parkinson’s disease: causes of discontinuation and subsequent treatment strategies. Neurol Sci. 2019;40(9):1917–23.
Drapier S, Eusebio A, Degos B, Vérin M, Durif F, Azulay JP, et al. Quality of life in Parkinson’s disease improved by apomorphine pump: the OPTIPUMP cohort study. J Neurol. 2016;263(6):1111–9.
Todorova A, Samuel M, Brown RG, Chaudhuri KR. Infusion therapies and development of impulse control disorders in advanced Parkinson disease: clinical experience after 3 years’ follow-up. Clin Neuropharmacol. 2015;38(4):132–4.
Fernandez HH, Boyd JT, Fung VSC, Lew MF, Rodriguez RL, Slevin JT, et al. Long-term safety and efficacy of levodopa-carbidopa intestinal gel in advanced Parkinson’s disease. Mov Disord. 2018;33(6):928–36.
Standaert DG, Aldred J, Anca-Herschkovitsch M, Bourgeois P, Cubo E, Davis TL, et al. DUOGLOBE: one-year outcomes in a real-world study of levodopa carbidopa intestinal gel for Parkinson’s disease. Movement Disord Clin Pract. 2021;8(7):1061–74.
Fabbri M, Zibetti M, Beccaria L, Merola A, Romagnolo A, Montanaro E, et al. Levodopa/carbidopa intestinal gel infusion and weight loss in Parkinson’s disease. Eur J Neurol. 2019;26(3):490–6.
Fabbri M, Zibetti M, Calandra-Buonaura G, Contin M, Sambati L, Mohamed S, et al. Levodopa/carbidopa intestinal gel long-term outcome in Parkinson’s disease: focus on dyskinesia. Movement Disord Clin Pract. 2020;7(8):930–9.
Marano M, Naranian T, di Biase L, Di Santo A, Poon YY, Arca R, et al. Complex dyskinesias in Parkinson patients on levodopa/carbidopa intestinal gel. Parkinsonism Relat Disord. 2019;69:140–6.
Merola A, Romagnolo A, Zibetti M, Bernardini A, Cocito D, Lopiano L. Peripheral neuropathy associated with levodopa-carbidopa intestinal infusion: a long-term prospective assessment. Eur J Neurol. 2016;23(3):501–9.
Romagnolo A, Merola A, Artusi CA, Rizzone MG, Zibetti M, Lopiano L. Levodopa-induced neuropathy: a systematic review. Movement Disord Clin Pract. 2019;6(2):96–103.
Rosebraugh M, Liu W, Neenan M, Facheris MF. Foslevodopa/foscarbidopa is well tolerated and maintains stable levodopa and carbidopa exposure following subcutaneous infusion. J Parkinsons Dis. 2021;11(4):1695–702.
Giladi N, Gurevich T, Djaldetti R, Adar L, Case R, Leibman-Barak S, et al. ND0612 (levodopa/carbidopa for subcutaneous infusion) in patients with Parkinson’s disease and motor response fluctuations: a randomized, placebo-controlled phase 2 study. Parkinsonism Relat Disord. 2021;91:139–45.
Olanow CW, Espay AJ, Stocchi F, Ellenbogen AL, Leinonen M, Adar L, et al. Continuous subcutaneous levodopa delivery for Parkinson’s disease: a randomized study. J Parkinsons Dis. 2021;11(1):177–86.
Soileau MSM, Aldred J, Budur K, Fisseha N, Fung V, Jeong A, Kimber T, Klos T, Litvan I, O’Neill D, Robieson W, Standaert D, Talapala S, OkeanisVaou E, Zheng H, Facheris M, Hauser R. Efficacy and safety of continuous subcutaneous foslevodopa/foscarbidopa in advanced Parkinson’s disease: results from a randomised, double-blind, active-controlled, phase 3 trial. Lancet Neurol. 2022;21(12):1099–109 (In Press).
Warren Olanow C, Torti M, Kieburtz K, Leinonen M, Vacca L, Grassini P, et al. Continuous versus intermittent oral administration of levodopa in Parkinson’s disease patients with motor fluctuations: a pharmacokinetics, safety, and efficacy study. Mov Disord. 2019;34(3):425–9.
Fabbri M, Rosa MM, Ferreira JJ. Adjunctive therapies in Parkinson’s disease: how to choose the best treatment strategy approach. Drugs Aging. 2018;35(12):1041–54.
Hauser RA, Auinger P. Determination of minimal clinically important change in early and advanced Parkinson’s disease. Mov Disord. 2011;26(5):813–8.
Hauser RA, Gordon MF, Mizuno Y, Poewe W, Barone P, Schapira AH, et al. Minimal clinically important difference in Parkinson’s disease as assessed in pivotal trials of pramipexole extended release. Parkinson’s Dis. 2014;2014: 467131.
Deuschl G, Antonini A, Costa J, Śmiłowska K, Berg D, Corvol JC, et al. European academy of neurology/movement disorder society-European section guideline on the treatment of Parkinson’s disease: I. Invasive therapies. Mov Disord. 2022;37(7):1360–74.
Krack P, Volkmann J, Tinkhauser G, Deuschl G. Deep brain stimulation in movement disorders: from experimental surgery to evidence-based therapy. Mov Disord. 2019;34(12):1795–810.
Rolston JD, Englot DJ, Starr PA, Larson PS. An unexpectedly high rate of revisions and removals in deep brain stimulation surgery: analysis of multiple databases. Parkinsonism Relat Disord. 2016;33:72–7.
Dafsari HS, Martinez-Martin P, Rizos A, Trost M, Dos Santos Ghilardi MG, Reddy P, et al. EuroInf 2: subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson’s disease. Mov Disord. 2019;34(3):353–65.
van der Kolk NM, de Vries NM, Kessels RPC, Joosten H, Zwinderman AH, Post B, et al. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: a double-blind, randomised controlled trial. Lancet Neurol. 2019;18(11):998–1008.
Schenkman M, Moore CG, Kohrt WM, Hall DA, Delitto A, Comella CL, et al. Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol. 2018;75(2):219–26.
Zhen K, Zhang S, Tao X, Li G, Lv Y, Yu L. A systematic review and meta-analysis on effects of aerobic exercise in people with Parkinson’s disease. NPJ Parkinson’s Dis. 2022;8(1):146.
Acknowledgements
Funding
No funding was received for this study or for the publication of this article.
Author Contributions
Conceptualization: Margherita Fabbri and Raquel Barbosa; Methodology: Margherita Fabbri and Olivier Rascol; Writing—original draft preparation: Margherita Fabbri and Raquel Barbosa; Writing—review: Olivier Rascol.
Disclosures
Margherita Fabbri declares the following conflicts of interest: honoraria to speak: BIAL and AbbVie; Consultancy/ LVL Médicale. Raquel Barbosa declares the following conflicts of interest: financial support by Fundação para a Ciência e Tecnologia (FCT) through a Ph.D. Scholarship (SFRH/BD/143797/2019) and Prémio João Lobo Antunes by Santa Casa da Misericórdia de Lisboa. Olivier Rascol declares the following conficts of interest: advisory board and consultancy: BIAL; advisory boards and consultancy: AbbVie, Adamas, Acorda, Addex, AlzProtect, Apopharma, Astrazeneca, Axovant, Biogen, Britannia, Buckwang, Cerespir, Clevexel, Denali, INC Reasearch, Lundbeck, Lupin, Merck, MundiPharma, Neuratris, Neuroderm, Novartis, ONO Pharma, Osmotica, Parexel, Pfzer, Prexton Therapeutics, Quintiles, Roche, Sanof, Servier, Sunovion, Théranexus, Takeda, Teva, UCB, Vectura, Watermark Research, XenoPort, XO, and Zambon; grant: Agence Nationale de la Recherche (ANR), CHU de Toulouse, France-Parkinson, INSERM-DHOS Recherche Clinique Translationnelle, MJFox Foundation, Programme Hospitalier de Recherche Clinique, European Commission (FP7, H2020), and Cure Parkinson IK; other: grant to participate in a symposium and contribute to the review of an IPMDS article.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original online version of this article was revised to correct an entry in Table 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fabbri, M., Barbosa, R. & Rascol, O. Off-time Treatment Options for Parkinson’s Disease. Neurol Ther 12, 391–424 (2023). https://doi.org/10.1007/s40120-022-00435-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00435-8