Abstract
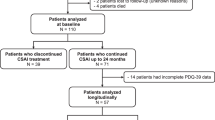
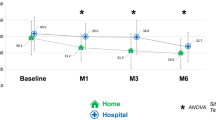
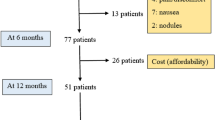
To report on OPTIPUMP, a cohort study, investigating the impact in real-life clinical settings of continuous subcutaneous apomorphine infusion (CSAI) on the quality of life (HRQoL) of patients with Parkinson’s disease. OPTIPUMP was a prospective, open-label, observational cohort study involving 30 investigational sites in France. CSAI was proposed as part of routine clinical care to patients aged ≥18 years, in absence of dementia, with a PD diagnosis and based on the presence of motor fluctuations not controlled by oral treatments. The impact of APO-pump on quality of life was evaluated as the difference in PDQ-39 scores between the initiation treatment and the follow-up visit after 6 months’ treatment. All adverse events were recorded. Hyper- and hypodopaminergic behavioral tolerance was assessed on the Ardouin Scale of Behavior in Parkinson’s Disease. Between September 2011 and January 2013, we enrolled 142 patients: 42 patients were withdrawn due to pump removal (33), death (4), lost of follow-up (4), no available data (1). 100 completed the study. At 6 months, their HRQoL had significantly improved (p = 0.011), as had their total UPDRS score (p < 0.001). Regarding the safety profile, Ardouin scale scores indicated that their hyperdopaminergic behaviors had not increased. CSAI had a favorable impact on HRQoL, with benefits outweighing risks. The analysis of the withdrawn patients highlights the heterogeneity of the use of the pump having an impact on its efficacy and tolerability.


Similar content being viewed by others
References
Colzi A, Turner K, Lees AJ (1998) Continuous subcutaneous waking day apomorphine in the long term treatment of levodopa induced interdose dyskinesias in Parkinson’s disease. J Neurol Neurosurg Psychiatry 64:573–576
García Ruiz PJ, Sesar Ignacio A, Ares Pensado B, Castro García A, Alonso Frech F, Alvarez López M, Arbelo González J, Baiges Octavio J, Burguera Hernández JA, Calopa Garriga M, Campos Blanco D, Castaño García B, Carballo Cordero M, Chacón Peña J, Espino Ibáñez A, Gorospe Onisalde A, Giménez-Roldán S, Granés Ibáñez P, Hernández Vara J, Ibáñez Alonso R, Jiménez Jiménez FJ, Krupinski J, Kulisevsky Bojarsky J, Legarda Ramírez I, Lezcano García E, Martínez-Castrillo JC, Mateo González D, Miquel Rodríguez F, Mir P, Muñoz Fargas E, Obeso Inchausti J, Olivares Romero J, Olivé Plana J, Otermin Vallejo P, Pascual Sedano B, de Colosía Pérez, Rama V, Pérez López-Fraile I, Planas Comes A, Puente Periz V, Rodríguez Oroz MC, Sevillano García D, Solís Pérez P, Suárez Muñoz J, Vaamonde Gamo J, Valero Merino C, Valldeoriola Serra F, Velázquez Pérez JM, Yáñez Baña R, Zamarbide Capdepon I (2008) Efficacy of long-term continuous subcutaneous apomorphine infusion in advanced Parkinson’s disease with motor fluctuations: a multicenter study. Mov Disord 23:1130–1136
Manson AJ, Turner K, Lees AJ (2002) Apomorphine monotherapy in the treatment of refractory motor complications of Parkinson’s disease: long-term follow-up study of 64 patients. Mov Disord 17:1235–1241
Pietz K, Hagell P, Odin P (1998) Subcutaneous apomorphine in late stage Parkinson’s disease: a long term follow up. J Neurol Neurosurg Psychiatry 65:709–716
Stibe CM, Lees AJ, Kempster PA, Stern GM (1988) Subcutaneous apomorphine in parkinsonian on-off oscillations. Lance 1:403–406
Di Rosa AE, Epifanio A, Antonini A, Stocchi F, Martino G, Di Blasi L, Tetto A, Basile G, Imbesi D, La Spina P, Di Raimondo G, Morgante L (2003) Continuous apomorphine infusion and neuropsychiatric disorders: a controlled study in patients with advanced Parkinson’s disease. Neurol Sci 24:174–175
Katzenschlager R, Hughes A, Evans A, Manson AJ, Hoffman M, Swinn L, Watt H, Bhatia K, Quinn N, Lees AJ (2005) Continuous subcutaneous apomorphine therapy improves dyskinesias in Parkinson’s disease: a prospective study using single-dose challenges. Mov Disord 20:151–157
Deleu D, Hanssens Y, Northway MG (2004) Subcutaneous apomorphine: an evidence-based review of its use in Parkinson’s disease. Drugs Aging 21:687–709
Peto V, Fitzpatrick R, Jenkinson C (1997) Self-reported health status and access to health services in a community sample with Parkinson’s disease. Disabil Rehabil 19:97–103
Schrag A, Jahanshahi M, Quinn N (2000) What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry 69:308–312
Rambour M, Moreau C, Salleron J, Devos D, Kreisler A, Mutez E, Simonin C, Annic A, Dujardin K, Destée A, Defebvre L (2014) Continuous subcutaneous infusion of apomorphine in Parkinson’s disease: retrospective analysis of a series of 81 patients. Rev Neurol 170:205–215
Martinez-Martin P, Reddy P, Antonini A, Henriksen T, Katzenschlager R, Odin P, Todorova A, Naidu Y, Tluk S, Chandiramani C, Martin A, Chaudhuri KR (2011) Chronic subcutaneous infusion therapy with apomorphine in advanced Parkinson’s disease compared to conventional therapy: a real life study of non motor effect. J Parkinsons Dis 1:197–203
Martinez-Martin P, Reddy P, Katzenschlager R, Antonini A, Todorova A, Odin P, Henriksen T, Martin A, Calandrella D, Rizos A, Bryndum N, Glad A, Dafsari HS, Timmermann L, Ebersbach G, Kramberger MG, Samuel M, Wenzel K, Tomantschger V, Storch A, Reichmann H, Pirtosek Z, Trost M, Svenningsson P, Palhagen S, Volkmann J, Chaudhuri KR (2015) EuroInf: a multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson’s disease. Mov Disord 30:510–516
Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51:745–752
Peto V, Jenkinson C, Fitzpatrick R, Greenhall R (1995) The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res 4:241–248
Guy W (1976) ECDEU assessment manual for psychopharmacology. US Department of Health, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration. Rockville, MD
Fahn S, Elton RL (1987) In: Fahn S, Marsden CD, Calne D (eds) Recent development in Parkinson’s disease. MacMillan Healthcare Information, Florham Park, pp 153–163
Schrag A, Sampaio C, Counsell N, Poewe W (2006) Minimal clinically important change on the unified Parkinson’s disease rating scale. Mov Disord 21:1200–1207
Ardouin C, Chéreau I, Llorca PM, Lhommée E, Durif F, Pollak P, Krack P, groupe évaluation comportementale de la maladie de Parkinson (2009) Assessment of hyper- and hypodopaminergic behaviors in Parkinson’s disease. Rev Neurol 165:845–856
Rieu I, Martinez-Martin P, Pereira B, De Chazeron I, Verhagen Metman L, Jahanshahi M, Ardouin C, Chéreau I, Brefel-Courbon C, Ory-Magne F, Klinger H, Peyrol F, Schupbach M, Dujardin K, Tison F, Houeto JL, Krack P, Durif F (2015) International validation of a behavioral scale in Parkinson’s disease without dementia. Mov Disord 30:705–713
Frankel JP, Lees AJ, Kempster PA, Stern GM (1990) Subcutaneous apomorphine in the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry 53:96–101
Morgante L, Basile G, Epifanio A, Spina E, Antonini A, Stocchi F, Di Rosa E, Martino G, Marconi R, La Spina P, Nicita-Mauro V, Di Rosa AE (2004) Continuous apomorphine infusion (CAI) and neuropsychiatric disorders in patients with advanced Parkinson’s disease: a follow-up of 2 years. Arch Gerontol Geriatr Suppl 9:291–296
Senek M, Nyholm D (2014) Continuous drug delivery in Parkinson’s disease. CNS Drugs 28:19–27
Lawrence AD, Evans AH, Lees AJ (2003) Compulsive use of dopamine replacement therapy in Parkinson’s disease: reward systems gone awry? Lancet Neurol 2:595–604
Chaudhuri KR, Healy DG, Schapira AH (2006) Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 5:235–245
Acknowledgments
The authors are indebted to Sébastien Woynar for his experienced participation in the organization of the study and technical support, Sara Calmanti for critical readings, manuscript editing and submission, and Elisabeth Portier for advice in manuscript editing.
Sophie Arguilliere, Stéphanie Bannier, Philippe Busson, Valérie Chauvire, Théodor Danaila, Pascal Derkinderen, Anne Doe De Maindreville, Frédérique Fluchere, Eric Gaujard, Julien Gere, Olivier Ille, Paul Krack, Pierre Krystkowiak, Amélie Leblanc, Romain Lefaucheur, Alain Legout, David Maltete, Fabienne Ory, Aurélia Poujois, Nicolaîe Rasvan Gosporady, Alain Razafindramboa, Anne Salmon, François Tison, Fréderic Torny, Tatiana Witjas, Farid Yekhlef, Fabienne Zaetta.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Study funding
The study has been funded by Orkyn’ and Aguettant.
Conflicts of interest
Dr Sophie Drapier received speech honorarium from Teva and Medtronic; and served on scientific advisory boards for Aguettant and Britannia. Dr Alexandre Eusebio received honoraria from UCB, GE Healthcare, Aguettant, is in the advisory board for Orkyn and has received research grants from the French Ministry of Health and the Agence Nationale de la Recherche. Dr Bertrand Degos received research support from INSERM (COSSEC), patient’s association France Parkinson; received travel fundings from Novartis, Teva, Lundbeck, MERZ-pharma; received speech honorarium from Novartis and Medtronic; and served on scientific advisory boards for Aguettant. Dr Marc Vérin has served on the Scientific Advisory Board for Aguettant and Orkyn and received speech honorarium from Teva and Medtronic. Dr Franck Durif has served on the Scientific Advisory Board for Aguettant and Orkyn and received speech honorarium from Teva and Medtronic. Dr Jean Philippe Azulay received honoraria from Abbvie, Boehringer, UCB and is in the advisory board for Abbvie, Zambon, UCB. Dr François Viallet has served on the Scientific Advisory Board for Aguettant and Orkyn. Dr Tiphaine Rouaud received speech honorarium from Teva. Dr Caroline Moreau has served on the Scientific Advisory Board for Aguettant. Dr Luc Defebvre served on the Scientific Advisory Board for Novartis, Aguettant and Abbvie, and has received various honoraria from pharmaceutical companies (Abbott Abbvie and Boehringer) for consultancy and lectures on Parkinson’s disease at symposia. Dr Valerie Fraix received honorarium from Medtronic France. Dr Christine Tranchant received speech honorarium from Teva and served on scientific advisory boards for Allergan and Abbvie. Karine Andre reports no disclosure. Dr Christine Brefel Courbon served on the Scientific Advisory Board for Novartis, Aguettant, UCB, Medtronic and received PHRC grants from the French Ministry of Research. She has received various honoraria for consultancy and lectures at symposia from pharmaceutical companies. Dr Emmanuel Roze is the recipient of a grant “poste d’accueil” AP-HP/CNRS. He received research support from INSERM (COSSEC), AP-HP (DRC-PHRC), Fondation pour la Recherche sur le Cerveau (FRC), Merz-Pharma, Orkyn, IP santé, Ultragenyx; served on scientific advisory boards for Orkyn, Ultragenyx and Merz-pharma; received speech honorarium from Merz-pharma, Novartis, Ipsen-Pharma and Orkyn, received travel funding from Ipsen-Pharma, Teva, Abbvie, Merz-Pharma, Dystonia Europe, the Georgian Medical and Public Health Association the International Federation of Clinical Neurophysiology, and the Movement Disorders Society. Dr David Devos served on the Scientific Advisory Board for Novartis and Aguettant and has received PHRC grants from the French Ministry of Health and research funding from the ARSLA charity. He has received various honoraria from pharmaceutical companies for consultancy and lectures on Parkinson’s disease at symposia.
Additional information
OPTIPUMP Study Group. Members of the OPTIPUMP Study Group are present in Acknowledgements.
Electronic supplementary material
Below is the link to the electronic supplementary material.
415_2016_8106_MOESM1_ESM.docx
Supplementary Fig. 1. Treatment changes during the study (DOCX 34 kb)
415_2016_8106_MOESM2_ESM.docx
Supplementary Fig. 2. UPDRS scores at baseline and 6 months after CSAI initiation (DOCX 26 kb)
415_2016_8106_MOESM3_ESM.docx
Supplementary Table 1. Apomorphine doses and rates between CSAI initiation and 6-month follow up (DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Drapier, S., Eusebio, A., Degos, B. et al. Quality of life in Parkinson’s disease improved by apomorphine pump: the OPTIPUMP cohort study. J Neurol 263, 1111–1119 (2016). https://doi.org/10.1007/s00415-016-8106-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8106-3