Abstract
Introduction
In 1995, the use of autologous hematopoietic stem-cell transplantation (AHSCT), which was previously used to treat hematological tumors, was introduced for severe autoimmune diseases such as multiple sclerosis (MS). AHSCT has proven its safety over the past few years due to technical advances and careful patient selection in transplant centers. While most studies have reported that AHSCT led to decreased Expanded Disability Status Scale (EDSS) scores, some patients reported increased EDSS scores following the procedure. Given the contradictory results, we aimed to conduct a comprehensive systematic review and meta-analysis to investigate the efficacy and safety of AHSCT.
Methods
PubMed, Web of Science, and Scopus were searched in March 2022 using a predefined search strategy. We included cohort studies, clinical trials, case–control studies, and case series that investigated the efficacy or safety of AHSCT in patients with MS. PICO in the present study was defined as follows: problem or study population (P): patients with MS; intervention (I): AHSCT; comparison (C): none; outcome (O): efficacy and safety.
Results
After a two-step review process, 50 studies with a total of 4831 patients with MS were included in our study. Our analysis showed a significant decrease in EDSS score after treatment (standardized mean difference [SMD]: −0.48, 95% CI −0.75, −0.22). Moreover, the annualized relapse rate was also significantly reduced after AHSCT compared to the pretreatment period (SMD: −1.58, 95% CI −2.34, −0.78). The pooled estimate of progression-free survival after treatment was 73% (95% CI 69%, 77). Furthermore, 81% of patients with MS who received AHSCT remained relapse-free (95% CI 76%, 86%). Investigating event-free survival, which reflects the absence of any disease-related event, showed a pooled estimate of 63% (95% CI 54%, 73%). Also, the MRI activity-free survival was 89% (95% CI 84%) among included studies with low heterogeneity. New MRI lesions seem to appear in nearly 8% of patients who underwent AHSCT (95% CI 4%, 12%). Our meta-analysis showed that 68% of patients with MS experience no evidence of disease activity (NEDA) after AHSCT (95% CI 59%, 77). The overall survival after transplantation was 94% (95% CI 91%, 96%). In addition, 4% of patients died from transplant-related causes (95% CI 2%, 6%).
Conclusion
Current data encourages a broader application of AHSCT for treating patients with MS while still considering proper patient selection and transplant methods. In addition, with increasing knowledge and expertise in the field of stem-cell therapy, AHSCT has become a safer treatment approach for MS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Current data encourage a broader application of autologous hematopoietic stem-cell transplantation (AHSCT) for treating patients with multiple sclerosis (MS). |
Our analysis showed a significant decrease in the Expanded Disability Status Scale (EDSS) score and annualized relapse rate after treatment compared with the pretreatment period. |
Our meta-analysis showed that 68% of patients with MS experience no evidence of disease activity (NEDA) after AHSCT. |
Introduction
Multiple sclerosis (MS) is characterized by chronic inflammation, neurodegeneration, and immune-mediated responses of the central nervous system (CNS), leading to demyelination, gliosis, and axonal damage [1, 2]. MS can cause permanent disability, reduce the quality of life, and shorten life span. Over the past two decades, disease-modifying therapies (DMTs) have been developed and approved, all of which have different efficacy and safety profiles. There have been considerable benefits for patients with relapsing–remitting MS (RRMS), as well as reduced clinical relapse. Although DMTs had a marginal effect on disability progression in RRMS, they failed to achieve an acceptable outcome in other subtypes of MS, such as progressive and treatment-refractory types [3,4,5].
In 1995, the use of autologous hematopoietic stem-cell transplantation (AHSCT), previously approved to treat hematological tumors, was introduced for severe autoimmune diseases [6, 7]. AHSCT is designed to remove the impaired immune system and then regenerate new immune cells to prevent the recurrence of neuroinflammatory symptoms [8, 9]. Previous studies have demonstrated the benefits of AHSCT in providing longer-term remission than conventional therapies. Also, the effectiveness and safety of this treatment approach were reported in autoimmune disease, especially in patients with MS who had not responded to DMTs [10, 11]. A retrospective cohort study on 120 patients with MS treated with AHSCT demonstrated a significantly decreased relapse rate at 2 and 4 years of follow-up, as well as a decrease in magnetic resonance imaging (MRI) T2 lesions. The study reported that 93% of patients were relapse-free at 2 years and 87% at 4 years. Based on the findings of this study, AHSCT was capable of preventing an increase in the Expanded Disability Status Scale (EDSS) scores [12]. Another study in patients with RRMS reported that five out of ten cases had complete remission after AHSCT at the end of the 10 years of follow-up. Also, three cases demonstrated improvement, so there is the possibility of complete remission after AHSCT [13]. Burt et al.’s study with a sample population of around 500 reported that AHSCT was a beneficial one-time treatment for RRMS. In contrast, their results showed less effectiveness of AHSCT in newly diagnosed secondary progressive MS [14]. Nowadays, AHSCT is recognized as a rapid treatment for relapsing or progressive multiple sclerosis. As a result, the National Multiple Sclerosis Society has acknowledged AHSCT as a feasible treatment option for patients with MS with high disease activity, as evidenced by relapse rates and new MRI lesions, despite the use of second-line DMTs, or in those with contraindications to conventional treatments. Indeed, patients under 50 years of age whose disease duration is less than 10 years are the best candidates for AHSCT [15]. A previous systematic review and meta-analysis demonstrated that progression-free survival after AHSCT in patients with MS was 75%, and estimated disease activity-free survival was 61% after 48 months [16].
As MS is generally not a life-threatening disease, concerns over mortality rates have previously restricted AHSCT application to treat MS. However, AHSCT has proven its safety over the past few years due to technical advances and careful patient selection in transplant centers. Thus, studies have reported that AHSCT led to decreased EDSS scores in most cases, although some patients had increased EDSS scores following the procedure [17,18,19]. In light of these contradictory results, we aimed to conduct a comprehensive systematic review and meta-analysis to investigate the efficacy and safety of AHSCT.
Methods
We conducted this systematic review and meta-analysis following the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) checklist [20]. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Search Strategy
We performed a comprehensive literature search on PubMed, Scopus, and Web of Science in February 2022. The following terms were used in our search strategy: “Multiple Sclerosis” OR “Sclerosis, Multiple” OR “Sclerosis, Disseminated” OR “Disseminated Sclerosis” AND “autologous hematopoietic stem cell transplantation” OR “AHSCT” OR “stem cell.” A manual search of the reference lists of previous review studies was also performed to identify additional articles.
Eligibility Criteria
We included cohort studies, clinical trials, case–control studies, and case series that investigated the efficacy or safety of AHSCT in patients with MS. Conference abstracts that were indexed in PubMed, Scopus, or Web of Science were also screened. The studies that investigated other types of stem-cell therapy such as progenitor cells, embryonic stem cells, or programmed stem cells were excluded. Also, case reports and non-English studies were excluded. PICO in the present study was defined as follows. Problem or study population (P): patients with MS; intervention (I): AHSCT; comparison (C): none; outcome (O): efficacy and safety.
Study Selection
Two authors (N.R., F.A.) independently screened the titles and abstracts to identify relevant studies. The same investigators then reviewed the full text of the selected papers for final selection. Any disagreement was resolved by consultation with a third reviewer (F.N.).
Data Extraction
The same reviewers (N.R., F.A.) extracted the following data from the selected studies: study demographics, sample size, gender, mean disease duration, type of MS, regimen intensity, cell dosage, EDSS at baseline, annualized relapse rate (ARR) at baseline, and the endpoint results regarding the efficacy and safety of AHSCT. A combination of total body irradiation (TBI) plus anti-thymocyte globulin (ATG) (TBI/ATG) is considered a high-intensity conditioning regimen, while the intermediate-intensity regimen most commonly used is the BEAM (carmustine, etoposide, cytarabine, and melphalan) plus ATG (BEAM/ATG) according to the European Society for Blood and Marrow Transplantation (EBMT) classification. There was no considerable difference in the AHSCT procedure among studies.
Endpoint
EDSS after treatment, ARR after treatment, progression-free survival (PFS), relapse-free survival (RFS), event-free survival (EFS), MRI activity-free survival (MAFS), no evidence of disease activity (NEDA), incidence of new MRI lesions after treatment, overall survival (OS), and transplant-related mortality (TRM) were extracted as endpoint data. There was substantial heterogeneity in the follow-up duration among studies. We extracted the efficacy and safety outcomes by default 5 years after transplantation. In studies with a shorter follow-up duration, we extracted the data for the longest endpoint.
Quality Assessments
The quality of observational studies was assessed using the Newcastle–Ottawa scale (NOS) [21] and the Cochrane risk-of-bias assessment tool for clinical trials by two independent investigators (N.R., F.A.), and consulting the third investigator (F.N.).
Statistical Analysis
We used Stata 11.0 software (StataCorp LLC, College Station, TX, USA) for statistical analysis. The medians and interquartile range were converted to mean and standard deviation based on the Hozo et al. method [22]. A standardized mean difference (SMD) methodology was applied for EDSS and ARR. The other efficacy and safety outcomes were pooled with a random-effects model and a 95% confidence interval (CI). Also, subgroup analysis based on the type of study and regimen intensity was performed. The Cochrane Q test and I-squared (I2) statistic were used to evaluate the heterogeneity among included studies.
Results
Search Results
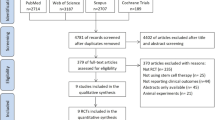
Our comprehensive search and manual addition yielded 1008 articles after duplicate removal (Fig. 1). Our initial title and abstract screening excluded 894 studies. In the end, 50 studies entered our meta-analysis and systematic review after full-text screening [12, 13, 17, 23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70].
A total of 4831 patients with MS, aged 26–60 years, were included in our study (Table 1). Among studies, 41 were cohort studies, eight were clinical trials, and one was a case series. The average quality score was 7.36 for observational studies, which is acceptable. For clinical trials, there was low publication bias (Supplementary 1 and 2). The detailed features of included studies are presented in Table 1.
Efficacy of AHSCT
We measured the efficacy of AHSCT with several outcomes including EDSS score change, ARR change, PFS, RFS, EFS, MAFS, NEDA, and incidence of new MRI lesions after treatment.
Our analysis showed a significant decrease in EDSS score after treatment (SMD: −0.48, 95% CI −0.75, −0.22; Q = 239.52, P < 0.00, I2 = 91.34%) (Fig. 2). The ARR was also significantly reduced after AHSCT relative to the pretreatment period (SMD: −1.58, 95% CI −2.34, −0.78; Q = 133.36, P < 0.00, I2 = 95.77%) (Fig. 3).
The pooled estimate of PFS after treatment was 73% (95% CI 69%, 77%; Q = 461.90, P < 0.00, I2 = 89.89%) (Supplementary 3). Furthermore, 81% of patients with MS who received AHSCT remained relapse-free (95% CI 76%, 86%; Q = 79.71, P < 0.00, I2 = 79.05%) (Supplementary 4). Investigation of EFS, which reflects the absence of any disease-related event, showed a pooled estimate of 63% (95% CI 54%, 73%; Q = 33.24, P < 0.00, I2 = 76.26%) (Supplementary 5). Also, the MAFS was 89% (95% CI 84%, 94%; Q = 3.25, P: 0.36, I2 = 26.66%) among included studies with low heterogeneity (Supplementary 6). New MRI lesions appeared in nearly 8% of patients who underwent AHSCT (95% CI 4%, 12%; Q = 5.31, P: 0.50, I2 = 0%) (Supplementary 7). Our meta-analysis showed that 68% of patients with MS experienced NEDA after AHSCT (95% CI 59%, 77%; Q = 37.93, P < 0.00, I2 = 75.97%) (Supplementary 8). The clinical outcomes are summarized in Fig. 4.
Safety of AHSCT
The overall survival after transplantation was 94% (95% CI 91%, 96%; Q = 93.60, P < 0.00, I2 = 83.92%) (Supplementary 9). In addition, 4% of patients died from transplant-related causes (95% CI 2%, 6%; Q = 89.13, P < 0.00, I2 = 93.21%) (Supplementary 10).
Discussion
Despite recent improvements in the application of AHSCT in MS, utilization of this treatment option is still limited. Many consider AHSCT among the final treatment strategies when other DMTs have failed [71]. In this systematic review, we aimed to address the lack of evidence supporting the confident application of AHSCT for patients with MS and to present a better view of the prospective benefits and potential risks.
The primary outcome measures for the efficacy of AHSCT were EDSS score change and ARR change. Regarding our analysis, both of these outcome measures showed reductions as a result of AHSCT. The decrease in the EDSS score is in line with previous meta-analyses, confirming the therapeutic application of AHSCT for halting the progression of MS [16, 72]. The reduction seen in ARR is also similar to the previous meta-analysis by Sormani et al., supporting the application of AHSCT in patients with MS with recurring relapses. It was previously shown that patients with RRMS are the most likely to benefit from AHSCT, besides having minimal transplant-related adverse effects compared with other MS subtypes [73, 74].
Based on our results, pooled estimates for PFS, RFS, and EFS showed promising results, confirming the effectiveness of AHSCT as a one-time and long-term treatment option for patients with MS. We also found slight but nonsignificant improvements in MAFS and incidence of new MRI lesions after treatment. Compared with other DMTs such as mitoxantrone (MTX), natalizumab, and alemtuzumab, AHSCT has shown better outcomes in controlling the progression and relapse of MS symptoms, in addition to achieving more extended periods of NEDA [70, 75, 76]. Currently the BEAT-MS (NCT04047628) trial is aiming to provide a comparison of the best available therapy versus AHSCT, though it is still in the patient recruitment stage. Further clinical trials are needed to elucidate a precise head-to-head comparison of these approaches.
We determined safety outcomes for AHSCT by overall survival and TRM. Contrary to previous findings, we found relatively high TRM. The initially high TRM of 3.6% decreased to 0.3% in studies post-2005 due to better patient selection, the use of proper regimens for immunoablation, and improved transplant techniques [73, 77, 78]. However, long-term outcomes measured by our analysis indicate higher TRM, raising a primary concern for AHSCT use in MS. We considered the endpoint of all TRM mainly at the end of 5-year follow-up duration; however, previous studies have considered a 100-day post-transplantation period for assessing TRM. This disparity in the definition of TRM may explain the observed difference in TRM between our research and previous meta-analyses.
As AHSCT targets the immune system, it can lead to several adverse events secondary to immune suppression. One study found that 79% of early non-neurological adverse effects, including neutropenic fever, sepsis, infections, and viral reactivation, were secondary to immunosuppression. Also, neurotoxicity occurred in 26 of 149 patients within 60 days of transplantation [60]. Late adverse events such as malignancies can be expected. Another study reported malignancies in nine of 281 patients [55]. Further studies with long follow-up duration are needed to determine the risk of potential adverse events after AHSCT in patients with MS.
AHSCT seems to hold better potential for treating patients with MS with different disease courses, as it is mainly considered among the final treatment options, and patient selection for AHSCT is usually made after many failed DMTs. The relatively high TRM of AHSCT versus other DMTs may be linked with patient characteristics. For instance, patients receiving AHSCT tend to have a more aggressive course of disease [55, 73]. Also, all AHSCT patients need to be protected from vaccine-preventable diseases, and the emergence of the COVID-19 pandemic has complicated this procedure in recent years [77]. Thus, the need for studies investigating the efficacy and safety of earlier AHSCT administration as mentioned in the EBMT criteria has increased.
EBMT recently issued guidelines with detailed patient characteristics appropriate for receipt of AHSCT, including highly active RRMS, disease duration less than 10 years, EDSS score equal to or less than 5.5, and age younger than 45 years [78]. By considering these in patient recruitment, achieving a better perspective on the efficacy and safety of AHSCT as a result of earlier administration is possible.
Although some guidelines have recently changed the position of AHSCT for RRMS from a “clinical option” to a “standard of care,” its use is still typically reserved for later in the disease course. As a result of growing evidence, equal footing of AHSCT with second-line DMTs for patients with RRMS is suggested [79]. Considering the superior efficacy of AHSCT in establishing long-term suppression of disease activity, it may be crucial to consider it before many of the second-generation DMTs to save time and prevent irreversible disease progression. However, this needs to be further investigated in large randomized controlled trials comparing the safety and efficacy of different DMTs with AHSCT in patients with distinct MS subtypes. There is a growing number of ongoing observational studies and clinical trials which can provide more evidence regarding the efficacy and safety of AHSCT in patients with MS and lead to optimization of this procedure (NCT numbers NCT03477500, NCT05029206, NCT04674280, NCT04047628).
Our study was limited in some aspects. First, due to the lack of studies focusing on specific subtypes of MS, we could not carry out a subgroup analysis. Also, there was relatively high heterogeneity between included studies, which led us to use random-effects analysis. Different patient characteristics, follow-up times, disease durations, subtypes of MS, conditioning regimens, and transplant techniques may have resulted in this heterogeneity.
Nevertheless, compared with a previous study by Ge et al. investigating the safety and efficacy of AHSCT in patients with MS [16], our study has several advantages. First, we investigated a greater number of efficacy and safety outcomes to give a comprehensive view of AHSCT in patients with MS. They excluded observational studies and only included 18 papers with a total of 731 patients, while we included 50 studies with a total of 4831 patients with MS. Furthermore, we used a more comprehensive search strategy in more medical databases to minimize missing papers and publication bias.
Conclusion
AHSCT is highly efficacious in treating patients with MS in multiple aspects, including preventing disease progression and relapse in addition to reducing inflammatory responses and associated CNS lesions. The few studies that have compared the efficacy of this treatment approach with currently available DMTs have reasonably indicated a better outcome. Although the patients enrolled in AHSCT trials are usually refractory to DMTs and develop a more aggressive disease course, comparisons with other DMT studies still show encouraging results. In addition, with the increasing knowledge and expertise in the field of stem-cell therapy, AHSCT has become a safer treatment approach for MS. Altogether, current data encourage a broader application of AHSCT for treating patients with MS while still considering proper patient selection and transplant methods.
References
Yamout BI, Alroughani R. Multiple Sclerosis. Semin Neurol. 2018;38(02):212–25.
McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA. 2021;325(8):765–79.
Wolinsky JS, Narayana PA, O’Connor P, Coyle PK, Ford C, Johnson K, et al. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann Neurol. 2007;61(1):14–24.
Wiendl H, Hohlfeld R. Multiple sclerosis therapeutics: unexpected outcomes clouding undisputed successes. Neurology. 2009;72(11):1008–15.
Giovannoni G, Lang S, Wolff R, Duffy S, Hyde R, Kinter E, et al. A Systematic review and mixed treatment comparison of pharmaceutical interventions for multiple sclerosis. Neurol Ther. 2020;9(2):359–74.
Currò D, Mancardi G. Autologous hematopoietic stem cell transplantation in multiple sclerosis: 20 years of experience. Neurol Sci. 2016;37(6):857–65.
Tyndall A, van Laar JM. Stem cell transplantation and mesenchymal cells to treat autoimmune diseases. La Presse MÚdicale. 2016;45(6):e159–69.
Alexander T, Farge D, Badoglio M, Lindsay JO, Muraro PA, Snowden JA. Hematopoietic stem cell therapy for autoimmune diseases–clinical experience and mechanisms. J Autoimmun. 2018;92:35–46.
Snowden JA. Rebooting autoimmunity with autologous HSCT. Blood. 2016;127(1):8–10.
Rebeiro P, Moore J. The role of autologous haemopoietic stem cell transplantation in the treatment of autoimmune disorders. Intern Med J. 2016;46(1):17–28.
Kelsey P, Oliveira M-C, Badoglio M, Sharrack B, Farge D, Snowden J. Haematopoietic stem cell transplantation in autoimmune diseases: from basic science to clinical practice. Curr Res Transl Med. 2016;64(2):71–82.
Nicholas RS, Rhone EE, Mariottini A, Silber E, Malik O, Singh-Curry V, et al. Autologous hematopoietic stem cell transplantation in active multiple sclerosis: a real-world case series. Neurology. 2021;97(9):e890–901.
Tolf A, Fagius J, Carlson K, Åkerfeldt T, Granberg T, Larsson EM, et al. Sustained remission in multiple sclerosis after hematopoietic stem cell transplantation. Acta Neurol Scand. 2019;140(5):320–7.
Burt RK, Han X, Quigley K, Helenowski IB, Balabanov R. Real-world application of autologous hematopoietic stem cell transplantation in 507 patients with multiple sclerosis. J Neurol. 2021;96(6):E817–E830.
Miller AE, Chitnis T, Cohen BA, Costello K, Sicotte NL, Stacom R. Autologous hematopoietic stem cell transplant in multiple sclerosis: recommendations of the National Multiple Sclerosis Society. JAMA Neurol. 2021;78(2):241–6.
Ge F, Lin H, Li Z, Chang T. Efficacy and safety of autologous hematopoietic stem-cell transplantation in multiple sclerosis: a systematic review and meta-analysis. Neurol Sci. 2019;40(3):479–87.
Chen B, Zhou M, Ouyang J, Zhou R, Xu J, Zhang Q, et al. Long-term efficacy of autologous haematopoietic stem cell transplantation in multiple sclerosis at a single institution in China. Neurol Sci. 2012;33(4):881–6.
Muraro PA, Pasquini M, Atkins HL, Bowen JD, Farge D, Fassas A, et al. Long-term outcomes after autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol. 2017;74(4):459–69.
Mancardi G, Sormani M, Di Gioia M, Vuolo L, Gualandi F, Amato M, et al. Autologous haematopoietic stem cell transplantation with an intermediate intensity conditioning regimen in multiple sclerosis: the Italian multi-centre experience. Mult Scler J. 2012;18(6):835–42.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7): e1000097.
Lo CK-L, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14(1):45.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13.
Abrahamsson SV, Angelini DF, Dubinsky AN, Morel E, Oh U, Jones JL, et al. Non-myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes IL-17 producing mucosal-associated invariant T cells in multiple sclerosis. Brain. 2013;136(Pt 9):2888–903.
Alping P, Burman J, Lycke J, Frisell T, Piehl F. Safety outcomes after treatment with alemtuzumab or autologous hematopoietic stem cell transplantation in multiple sclerosis patients. Multiple Scler J. 2020;26(3_Suppl):352–3.
Arruda LC, Clave E, Moins-Teisserenc H, Douay C, Farge D, Toubert A. Resetting the immune response after autologous hematopoietic stem cell transplantation for autoimmune diseases. Curr Res Transl Med. 2016;64(2):107–13.
Atkins HL, Bowman M, Allan D, Anstee G, Arnold DL, Bar-Or A, et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet. 2016;388(10044):576–85.
Chen B, Ouyang J, Zhou M, Shao XY, Xu Y, Zhou RF. Autologous hematopoietic stem cell transplantation in multiple sclerosis: long-term clinical outcome in China. Blood. 2011;118(21):883–4.
Blanco Y, Saiz A, Carreras E, Graus F. Autologous haematopoietic-stem-cell transplantation for multiple sclerosis. Lancet Neurol. 2005;4(1):54–63.
Boffa G, Massacesi L, Inglese M, Mariottini A, Capobianco M, Lucia M, et al. Long-term clinical outcomes of hematopoietic stem cell transplantation in multiple sclerosis. Neurology. 2021;96(8):E1215–E1226.
Bose G, Atkins HL, Bowman M, Freedman MS. Autologous hematopoietic stem cell transplantation improves fatigue in multiple sclerosis. Mult Scler. 2019;25(13):1764–72.
Bowen JD, Kraft GH, Wundes A, Guan Q, Maravilla KR, Gooley TA, et al. Autologous hematopoietic cell transplantation following high-dose immunosuppressive therapy for advanced multiple sclerosis: long-term results. Bone Marrow Transplant. 2012;47(7):946–51.
Burt RK, Balabanov R, Han XQ, Quigley K, Arnautovic I, Helenowski I, et al. Autologous hematopoietic stem cell transplantation for stiff-person spectrum disorder a clinical trial. Neurology. 2021;96(6):E817–30.
Casanova B, Jarque I, Gascón F, Hernández-Boluda JC, Pérez-Miralles F, de la Rubia J, et al. Autologous hematopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: comparison with secondary progressive multiple sclerosis. Neurol Sci. 2017;38(7):1213–21.
Daumer M, Griffith LM, Meister W, Nash RA, Wolinsky JS. Survival, and time to an advanced disease state or progression, of untreated patients with moderately severe multiple sclerosis in a multicenter observational database: relevance for design of a clinical trial for high dose immunosuppressive therapy with autologous hematopoietic stem cell transplantation. Mult Scler. 2006;12(2):174–9.
Darlington PJ, Stopnicki B, Touil T, Doucet JS, Fawaz L, Roberts ME, et al. Natural killer cells regulate Th17 cells after autologous hematopoietic stem cell transplantation for relapsing remitting multiple sclerosis. Front Immunol. 2018;9:834.
Das J, Snowden JA, Burman J, Freedman MS, Atkins H, Bowman M, et al. Autologous haematopoietic stem cell transplantation as a first-line disease-modifying therapy in patients with “aggressive” multiple sclerosis. Mult Scler. 2021;27(8):1198–204.
Dayama A, Bhargava R, Kurmi S, Jain S, Dua V. Autologous stem cell transplant in adult multiple sclerosis patients: a study from North India. Neurol India. 2020;68(2):454–7.
de Oliveira GL, Ferreira AF, Gasparotto EP, Kashima S, Covas DT, Guerreiro CT, et al. Defective expression of apoptosis-related molecules in multiple sclerosis patients is normalized early after autologous haematopoietic stem cell transplantation. Clin Exp Immunol. 2017;187(3):383–98.
Evdoshenko EP, Zubarovskaya LS, Zaslavsky LG, Skoromets AA, Alexeev SA, Stankevich JA, et al. The feasibility of high dose chemotherapy with autologous stem cell transplantation for multiple sclerosis. Cell Ther Transplant. 2011;2(6):284–292.
Farge D, Labopin M, Tyndall A, Fassas A, Mancardi GL, Van Laar J, et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years’ experience from the European Group for Blood and Marrow Transplantation Working Party on Autoimmune Diseases. Haematologica. 2010;95(2):284–92.
Fassas A, Passweg JR, Anagnostopoulos A, Kazis A, Kozak T, Havrdova E, et al. Hematopoietic stem cell transplantation for multiple sclerosis. A retrospective multicenter study. J Neurol. 2002;249(8):1088–97.
Giedraitiene N, Kizlaitiene R, Peceliunas V, Griskevicius L, Kaubrys G. Selective cognitive dysfunction and physical disability improvement after autologous hematopoietic stem cell transplantation in highly active multiple sclerosis. Sci Rep. 2020;10(1):21286.
Gualandi F, Bruno B, Van Lint MT, Luchetti S, Uccelli A, Capello E, et al. Autologous stem cell transplantation for severe autoimmune diseases: a 10-year experience. Ann N Y Acad Sci. 2007;1110:455–64.
Guillaume-Jugnot P, Badoglio M, Labopin M, Terriou L, Yakoub-Agha I, Martin T, et al. Autologous haematopoietic stem cell transplantation (AHSCT) in autoimmune disease adult patients in France: analysis of the long-term outcome from the French Society for Bone Marrow Transplantation and Cellular Therapy (SFGM-TC). Clin Rheumatol. 2019;38(5):1501–11.
Guimarães FA, Oliveira-Cardoso EA, Mastropietro AP, Voltarelli JC, Santos MA. Impact of autologous hematopoetic stem cell transplantation on the quality of life of patients with multiple sclerosis. Arq Neuropsiquiatr. 2010;68(4):522–7.
Hamerschlak N, Rodrigues M, Moraes DA, Oliveira MC, Stracieri A, Pieroni F, et al. Brazilian experience with two conditioning regimens in patients with multiple sclerosis: BEAM/horse ATG and CY/rabbit ATG. Bone Marrow Transplant. 2010;45(2):239–48.
Häußler V, Ufer F, Pöttgen J, Wolschke C, Friese MA, Kröger N, et al. aHSCT is superior to alemtuzumab in maintaining NEDA and improving cognition in multiple sclerosis. Ann Clin Transl Neurol. 2021;8(6):1269–78.
Karnell FG, Lin D, Motley S, Duhen T, Lim N, Campbell DJ, et al. Reconstitution of immune cell populations in multiple sclerosis patients after autologous stem cell transplantation. Clin Exp Immunol. 2017;189(3):268–78.
Krasulová E, Trneny M, Kozák T, Vacková B, Pohlreich D, Kemlink D, et al. High-dose immunoablation with autologous haematopoietic stem cell transplantation in aggressive multiple sclerosis: a single centre 10-year experience. Mult Scler. 2010;16(6):685–93.
Kvistad SAS, Lehmann AK, Trovik LH, Kristoffersen EK, Bø L, Myhr KM, et al. Safety and efficacy of autologous hematopoietic stem cell transplantation for multiple sclerosis in Norway. Mult Scler. 2020;26(14):1889–97.
Mariottini A, Innocenti C, Forci B, Magnani E, Mechi C, Barilaro A, et al. Safety and efficacy of autologous hematopoietic stem-cell transplantation following natalizumab discontinuation in aggressive multiple sclerosis. Eur J Neurol. 2019;26(4):624–30.
Massey J. Autologous haematopoietic stem cell transplantation (AHSCT) in relapse remitting (RR-MS) and secondary progressive multiple sclerosis (SP-MS): a phase II trial from St Vincent’s Hospital. Sydney Australia Multiple Sclerosis Journal. 2017;23:594.
Mancardi GL, Sormani MP, Di Gioia M, Vuolo L, Gualandi F, Amato MP, et al. Autologous haematopoietic stem cell transplantation with an intermediate intensity conditioning regimen in multiple sclerosis: the Italian multi-centre experience. Mult Scler. 2012;18(6):835–42.
Moore JJ, Massey JC, Ford CD, Khoo ML, Zaunders JJ, Hendrawan K, et al. Prospective phase II clinical trial of autologous haematopoietic stem cell transplant for treatment refractory multiple sclerosis. J Neurol Neurosurg Psychiatry. 2019;90(5):514–21.
Muraro PA, Martin R, Mancardi GL, Nicholas R, Sormani MP, Saccardi R. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat Rev Neurol. 2017;13(7):391–405.
Murrieta-Álvarez I, Cantero-Fortiz Y, León-Peña AA, Olivares-Gazca JC, Priesca-Marín JM, Ruiz-Delgado GJ, et al. The 1,000th transplant for multiple sclerosis and other autoimmune disorders at the hsct-méxico program: a myriad of experiences and knowledge. Front Neurol. 2021;12:842–852.
Nash RA, Hutton GJ, Racke MK, Popat U, Devine SM, Steinmiller KC, et al. High-dose immunosuppressive therapy and autologous HCT for relapsing-remitting MS. Neurology. 2017;88(9):842–52.
Ni XS, Ouyang J, Zhu WH, Wang C, Chen B. Autologous hematopoietic stem cell transplantation for progressive multiple sclerosis: report of efficacy and safety at three yr of follow up in 21 patients. Clin Transplant. 2006;20(4):485–9.
Ruiz-Argüelles A, Gastélum-Cano JM, Méndez-Huerta MA, Rodríguez-Gallegos AB, Ruiz-Argüelles GJ. Glomerular filtration rate in patients with multiple sclerosis undergoing stem cell transplantation and treated with cyclophosphamide. Lab Med. 2019;50(1):42–6.
Saccardi R, Kozak T, Bocelli-Tyndall C, Fassas A, Kazis A, Havrdov E, et al. Autologous stem cell transplantation for progressive multiple sclerosis: update of the European Group for Blood and Marrow Transplantation autoimmune diseases working party database. Mult Scler. 2006;12(6):814–23.
Saiz A, Blanco Y, Carreras E, Berenguer J, Rovira M, Pujol T, et al. Clinical and MRI outcome after autologous hematopoietic stem cell transplantation in MS. Neurology. 2004;62(2):282–4.
Shevchenko JL, Kuznetsov AN, Ionova TI, Melnichenko VY, Fedorenko DA, Kurbatova KA, et al. Long-term outcomes of autologous hematopoietic stem cell transplantation with reduced-intensity conditioning in multiple sclerosis: physician’s and patient’s perspectives. Ann Hematol. 2015;94(7):1149–57.
Sousa ADA, Malmegrim KCR, Panepucci RA, Brum DS, Barreira AA, Dos Santos AC, et al. Autologous haematopoietic stem cell transplantation reduces abnormalities in the expression of immune genes in multiple sclerosis. Clin Sci. 2015;128(2):111-U57.
Su L, Xu J, Ji BX, Wan SG, Lu CY, Dong HQ, et al. Autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Int J Hematol. 2006;84(3):276–81.
Wiberg A, Olsson-Strömberg U, Herman S, Kultima K, Burman J. Profound but transient changes in the inflammatory milieu of the blood during autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2020;26(1):50–7.
Zhukovsky C, Sandgren S, Silfverberg T, Einarsdottir S, Tolf A, Landtblom AM, et al. Autologous haematopoietic stem cell transplantation compared with alemtuzumab for relapsing-remitting multiple sclerosis: an observational study. J Neurol Neurosurg Psychiatry. 2021;92(2):189–94.
Arruda LC, Lorenzi JC, Sousa AP, Zanette DL, Palma PV, Panepucci RA, et al. Autologous hematopoietic SCT normalizes miR-16, -155 and -142-3p expression in multiple sclerosis patients. Bone Marrow Transplant. 2015;50(3):380–9.
Xu J, Ji BX, Su L, Dong HQ, Sun WL, Wan SG, et al. Clinical outcome of autologous peripheral blood stem cell transplantation in opticospinal and conventional forms of secondary progressive multiple sclerosis in a Chinese population. Ann Hematol. 2011;90(3):343–8.
Samijn JPA, te Boekhorst PAW, Mondria T, van Doorn PA, Flach HZ, van der Meché FGA, et al. Intense T cell depletion followed by autologous bone marrow transplantation for severe multiple sclerosis. J Neurol Neurosurg Psychiatry. 2006;77(1):46–50.
Burt RK, Balabanov R, Burman J, Sharrack B, Snowden JA, Oliveira MC, et al. Effect of nonmyeloablative hematopoietic stem cell transplantation vs continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA. 2019;321(2):165–74.
Yamout B, Sahraian M, Bohlega S, Al-Jumah M, Goueider R, Dahdaleh M, et al. Consensus recommendations for the diagnosis and treatment of multiple sclerosis: 2019 revisions to the MENACTRIMS guidelines. Mult Scler Relat Disord. 2020;37: 101459.
Zhang P, Liu B. Effect of autologous hematopoietic stem cell transplantation on multiple sclerosis and neuromyelitis optica spectrum disorder: a PRISMA-compliant meta-analysis. Bone Marrow Transplant. 2020;55(10):1928–34.
Sormani MP, Muraro PA, Schiavetti I, Signori A, Laroni A, Saccardi R, et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis: a meta-analysis. Neurology. 2017;88(22):2115–22.
Lycke J, Lenhoff S. Intensive immunosuppression followed by autologous hematopoietic stem cell transplantation for the treatment of multiple sclerosis. Ther Adv Neurol Disord. 2020;13:1756286420929467.
Boffa G, Lapucci C, Sbragia E, Varaldo R, Raiola AM, Currò D, et al. Aggressive multiple sclerosis: a single-centre, real-world treatment experience with autologous haematopoietic stem cell transplantation and alemtuzumab. Eur J Neurol. 2020;27(10):2047–55.
Mancardi GL, Sormani MP, Gualandi F, Saiz A, Carreras E, Merelli E, et al. Autologous hematopoietic stem cell transplantation in multiple sclerosis: a phase II trial. Neurology. 2015;84(10):981–8.
Cencioni MT, Genchi A, Brittain G, de Silva TI, Sharrack B, Snowden JA, et al. Immune reconstitution following autologous hematopoietic stem cell transplantation for multiple sclerosis: a review on behalf of the EBMT Autoimmune Diseases Working Party. Front Immunol. 2021;12: 813957.
Sharrack B, Saccardi R, Alexander T, Badoglio M, Burman J, Farge D, et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE). Bone Marrow Transplant. 2020;55(2):283–306.
Bertolotto A, Martire S, Mirabile L, Capobianco M, De Gobbi M, Cilloni D. Autologous hematopoietic stem cell transplantation (AHSCT): standard of care for relapsing-remitting multiple sclerosis patients. Neurol Ther. 2020;9(2):197–203.
Bonechi E, Aldinucci A, Mazzanti B, di Gioia M, Repice AM, Manuelli C, Saccardi R, Massacesi L, Ballerini C. Increased CXCL10 expression in MS MSCs and monocytes is unaffected by AHSCT. Ann Clin Transl Neurol. 2014;1(9):650–8. https://doi.org/10.1002/acn3.92
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
FN, OM, KP, AZ & MAA: designed the study, analyzed the data, and wrote the paper; FN, NR & FA: collected data, analyzed and interpreted the data, and wrote the draft version of the manuscript. The manuscript was revised and approved by all authors.
Disclosures
Fardin Nabizadeh, Kasra Pirahesh, Nazanin Rafiei, Fatemeh Afrashteh, Mona Asghari Ahmadabad, Aram Zabeti, and Omid Mirmosayyeb declare no conflict of interest regarding the publication of this paper.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets analyzed during the current study are available upon request with no restriction.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nabizadeh, F., Pirahesh, K., Rafiei, N. et al. Autologous Hematopoietic Stem-Cell Transplantation in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Neurol Ther 11, 1553–1569 (2022). https://doi.org/10.1007/s40120-022-00389-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00389-x