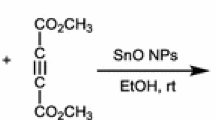
Abstract
A new protocol for the synthesis of 2H-indazolo[2,1-b]phthalazine-trione derivatives is described via a one-pot three-component condensation reaction of phthalhydrazide, dimedone or 1,3-cyclohexanedione and aromatic aldehydes catalyzed by SnO2 nanoparticles as a heterogeneous catalyst under solvent-free conditions. The SnO2 nanoparticles (NPs) were characterized by FT-IR, X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM) and energy dispersive X-ray analyzer (EDAX). The advantages of the protocol are the shorter reaction time, simple work-up procedure and reusable catalyst.
Graphical Abstract

Similar content being viewed by others
Introduction
Generally, nitrogen containing heterocyclic compounds have a wide range of pharmacological and clinical applications [1,2,3,4,5,6]. Among them, nitrogen incorporated phthalazine containing bridgehead hydrazine heterocyclic compounds are highly desirable drugs with anticonvulsant [7], cardiotonic [8], vasorelaxant [9], antimicrobial [10], antifungal [11], anticancer [12] and anti-inflammatory [13] activities. In addition, phthalazine derivatives also have excellent optical properties [14, 15]. Due to the notable significances, we would like to establish a new facile protocol for the synthesis of phthalazine scaffolds.
In recent years, the literature reports reveal that only a limited number of solvent-free protocols are available for the synthesis of phthalazine derivatives using the condensation reaction of phthalhydrazide, dimedone, and aromatic aldehydes with various catalysts [16,17,18,19,20,21,22,23,24,25]. However, the reported methods have some limitations such as non-biodegradable and expensive metal catalysts, corrosive reaction media and longer reaction times due to which new synthetic protocols may be suggested for the synthesis of phthalazine derivatives via one-pot multicomponent reactions. Multicomponent reactions (MCRs) are more essential to construct the complex organic molecule in a single step without isolation of any intermediates [29, 30]. MCRs with heterogeneous catalyst and solvent-free process are more attractive to the researchers, because they will minimize the waste and usage of hazardous chemicals [31, 32]. Moreover, the heterogeneous catalysts have more enrapture to the researchers because of their selectivity and reactivity in organic transformations [33,34,35] and they provide more advantages over homogeneous catalysts due to the more active sites. In addition, they are environmental friendly, cost effective, insoluble in solvents and reusable leading to easy work-up procedures. In general, the nanomaterials exhibit superior mechanical, thermal, chemical, electrical, optical and catalytic properties. Therefore, we extend our present research work by employing SnO2 nanoparticles as a heterogeneous catalyst for the synthesis of phthalazine derivatives. Tin oxide (SnO2) has a broad spectrum of applications which includes its use in electrochemical [36] and photo-catalytical processes [37], gas sensors [38, 39], optical electronic devices [40] and solar cells [41]. Additionally, the SnO2 NPs have an excellent catalytic behavior in the synthesis of various heterocyclic compounds [42,43,44,45].
The present research work utilizes SnO2 NPs as a reusable catalyst for the synthesis of 2H-indazolo[2,1-b]phthalazine-trione derivatives. To the best of our knowledge, there is no literature report available for the SnO2 NPs-catalyzed synthesis of 2H-indazolo[2,1-b]phthalazine-triones.
Experimental Methods
General
All reagents and common solvents were purchased from Loba chemie and Sigma-Aldrich and used without further purification. The products were isolated and characterized by physical and spectral analysis. 1H NMR and 13C NMR spectra were recorded on Bruker (DRX Avance-400) spectrometer in the presence of tetramethylsilane as an internal standard. The IR spectra were recorded on Bruker (ATR FT-IR) apparatus using Zn-Se technique. Reactions were monitored by TLC using precoated silica gel plates and melting points were determined on an electro thermal apparatus, and are uncorrected. Powder X-ray diffraction (XRD) was carried out on a Philips diffractometer of X’pert Company with monochromatized Cu Ka radiation (k = 1.5406 A˚). Field emission scanning electron microscopy (FE-SEM) and the X-ray dispersive analysis were recorded by FE-SEM (LEO 1455VP), operated at a 15 kV accelerating voltage.
General procedure for the preparation of the SnO2 nanoparticles
The SnO2 NPs were prepared by taking 2 g (0.1 M) of stannous chloride dihydrate (SnCl2·2H2O) in 50 ml of distilled water and dissolved. Simultaneously, an aqueous ammonia solution was added drop by drop under continuous stirring for 15–20 min while maintaining the pH of the solution between 9 and 11. The resulting gel was washed with deionized water until the pH reached 7, filtered and dried at 80 °C for 24 h to remove water. Then, the powdered sample was calcinated at 600 °C for 2 h and the finally obtained powder sample was characterized with FT-IR, powder X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM) and X-ray dispersive analyses.

General procedure for the synthesis of 2H-indazolo[2,1-b]phthalazine-trione derivatives
An equimolar mixture of phthalhydrazide (1.0 mmol), aromatic aldehyde (1.0 mmol), 5,5-dimethyl-1,3-cyclohexanedione or 1,3-cyclohexanedione (1.0 mmol) and 10 mol % of SnO2 NPs was magnetically stirred over an oil bath at 80 °C under solvent-free condition for an appropriate time. The completion of the reaction was monitored by TLC using ethyl acetate–hexane (1:1) as the eluent. After completion of the reaction, the reaction mass was cooled to room temperature and 3 ml of chloroform was added and stirred well. The product was soluble in chloroform and the catalyst was recovered by simple filtration. The catalyst was washed with 3 ml of acetone and dried at 100 °C. Finally, the recovered catalyst was subjected to the next run of the reaction. The solvent was removed under vacuum to get the crude product, which on further recrystallization with ethanol afforded the pure product (compared with literatures Ref. [16–18, 26–28]). The pure products were characterized with FT-IR, 1H and 13C NMR spectroscopic techniques.
Results and discussion
Characterization of SnO2 NPs
The SnO2 NPs were synthesized using an already reported procedure [38]. The synthesized SnO2 NPs were characterized by Fourier transform-infrared spectroscopy, powder X-ray diffraction, field emission scanning electron microscopy and energy dispersive X-ray spectroscopy.
FT-IR and XRD analysis
We have compared the FT-IR spectra of as-synthesized form and calcinated samples of SnO2 NPs (Fig. 1). Figure 1a shows a broad band at 3460.78 cm−1 which represents the presence of water molecule bound to Sn surface. The band at 1573.15 cm−1is due to the bending vibration of water molecules and the peak at 1423.15 cm−1represents the unreactive SnCl2. The band at 625 cm−1 indicates the Sn–OH. Furthermore, the calcinated sample has shown a band at 646.31 cm−1 corresponds to Sn–O–Sn and the band present at 553.94 cm−1 is due to the stretching vibration of Sn–O. The intensity of peaks due to water molecules is very much decreased in the case of calcinated sample. The observed results reveal that the Sn–O band is not present in the fresh sample. Figure 1c shows the IR spectrum of the SnO2 catalyst after the sixth run of the reaction in which there is no change in the peak position and intensity when compared to that of the calcinated catalyst supporting its reusability results. From the X-ray diffraction spectrum, the formations of SnO2 NPs are confirmed (Fig. 2). All the diffraction peaks are in good agreement with the standard (JCPDS No- 41-1445) which can be indexed as the tetragonal structure of SnO2 with lattice constant of a = 4.78, c = 3.17 and unit cell volume d = 72.66. No other diffraction peaks are observed in the XRD pattern that clearly indicates the purity of SnO2 NPs. The average crystallite size of the SnO2 NPs was calculated using Scherrer formula as, (D = kλ/βcosθ), where D is the average crystallite size, k is the Scherrer constant, λ is the wavelength of the X-ray beam, β is the (π/2) FWHM (Full width at half maximum) and θ is the diffraction angle. The average crystallite size of the SnO2 NPs was found to be 23.4 nm. Meanwhile, these results were in good agreement with the literature report [46].
FE-SEM and EDAX analysis
The field emission scanning electron microscopy (FE-SEM) that shows the morphology and size of the SnO2 NPs is presented in Fig. 3. The morphology and size of the individual particles could not be measured due to their extremely small size and heavy aggregation. In general, the formation of agglomerated nanoparticles can be explained by nucleation–aggregation–agglomeration–growth mechanism proposed by Rodriguez-Clemente et al. [47]. The particle size of the SnO2 NPs is in the range of 44–71.79 nm approximately and also the SnO2 NPs have been aggregated in spherical morphology. Finally, the energy dispersive X-ray spectrum exhibits the atomic weight % of tin 33.33 and 66.67% of oxygen present in the SnO2 NPs (Fig. 4).
Catalytic activity
The formation of SnO2 NPs made us to utilize the NPs as a catalyst for the one-pot synthesis of 2H-indazolo[2,1-b]phthalazine-triones under various reaction conditions. We have optimized the amount of catalyst required to catalyze the reaction where the temperature and solvent have also been screened. Initially, we have performed the model reaction with a mixture of 4-chlorobenzaldehyde (1 mmol), dimedone (1 mmol) and phthalhydrazide (1 mmol) without catalyst and solvent and found that there was no reaction even after five hours (Table 1, Entry 1) revealing the need of a catalyst to activate the reaction. By employing 20 mol % of various Lewis acid catalysts such as NiCl2.6H2O, CuCl2.2H2O, CaCl2.2H2O and MnCl2.4H2O for the reaction, the yield of the expected product was very low even after 60 min of the reaction (Table 1, Entries 2-5). Next, we investigated the reaction with 20 mol % of SnCl2·2H2O, where the moderate yield of the product was observed (Table 1, Entry 6). The model reaction when conducted at 80 °C with 10 mol % of SnO2 bulk led to prolonged reaction time with 72% yield (Table 1, Entry 7) which made us to standardize the reaction using SnO2 NPs. Using 5 mol % SnO2 NPs, the reaction provided 70% of the product with lesser reaction time when compared with other metal chlorides (Table 1, Entry 8) and 10 mol % of the SnO2 NPs catalyzed the reaction to an excellent yield of the product (Table 1, Entry 9) with shorter reaction time. Further increase in the mol % of SnO2 NPs did not improve the yield of the product (Table 1, Entries 10 and 11). Meanwhile, the model reaction was studied with 10 mol % of SnO2 NPs under various temperatures. At 80 and 90 °C, there was no change in the yield and reaction time, (Table 1, Entry 12) but the yield decreased at 70 or 60 °C (Table 1, entries 13 and 14).
With the optimized catalyst, solvent-mediated reaction did not succeed well which may be due to the interruption of solvent molecules for the direct contact between the reactants and the catalyst (Table 1, entries 15–17). We have compared the yield of the product and the reaction time with previous literature in Table 2, entries 1–10. Apparently, some of the reported catalysts afforded good yields but most of the catalysts were hazardous to the environment and the catalyst quantity needed was also more in addition to longer reaction time. The catalytic activity of SnO2 NPs may be due to the presence of more acidic (Sn4+) and the basic (O2−) surface sites [37] that could readily induce the organic transformations. We have concluded that 10 mol % of SnO2 NPs at 80 °C was more favorable under solvent-free condition to provide excellent yield of the target product with shorter reaction time (Table 1, entry 9) and the optimization results are presented in Table 1. The reusability of the catalyst was also successfully checked up to seven runs.
Using the above optimized conditions, we have taken various aromatic aldehydes having different electron withdrawing and donating substituents in the ortho, meta and para positions and cyclic diketones for the synthesis of 2H-indazolo[2,1-b]phthalazine-trione derivatives (Table 3). Almost all aldehydes provided good to excellent yields of the products irrespective of the position as well as electron donating and withdrawing nature of the group in the aryl aldehydes (Scheme 1).
The synthesized 2H-indazolo[2,1-b]phthalazine-trione derivatives were confirmed by 1H-NMR and 13C-NMR spectroscopic techniques. The 1H-NMR spectrum of the compound 4p exhibited a peak at δ 2.24–3.59 ppm which could confirm the presence of six methylene protons in the cyclohexenone ring and another singlet appeared at δ 3.75 ppm which indicates the presence of 4-OCH3 on the aromatic ring. The methine (C–H) proton has given a single peak at δ 6.42 ppm and the remaining aromatic protons exhibited peaks at δ 6.84–8.35 ppm. The 13C-NMR spectrum of the compound (4p) exhibited peak at δ 22.35–64.60 ppm which corresponds to the presence of aliphatic carbon in the cyclohexenone ring and the peak appeared at δ 114.11 ppm due to the presence of methine carbon. The characteristic peak of carbonyl carbon appeared at δ 192.62 ppm and the rest of the aromatic carbons appeared at δ 159.74–119.68 ppm.
The plausible reaction mechanism proposed for the synthesis of 2H-indazolo[2,1-b]phthalazine-trionederivatives is represented in Scheme 2. Initially, the SnO2 NPs catalyzed the Knoevenagel condensation reaction between dimedone (I) and aromatic aldehyde (II) providing III. This step is facilitated by the activation of carbonyl group in aromatic aldehyde by SnO2 NPs. Michael addition of phthalhydrazide to III provides IV which on cyclization afforded the target product (V).
Reusability of the catalyst
Next, we have also checked the reusability of SnO2 NPs. The reusability of the catalyst was investigated with the model reaction using an equimolar mixture of 4-chlorobenzaldehyde (1 mmol), dimedone (1 mmol) and phthalhydrazide (1 mmol) performed with 10 mol % of SnO2 NPs under solvent-free condition. After completion of the reaction, the reaction mass was cooled to room temperature and 3 ml of chloroform was added and stirred well. After that the product soluble in chloroform was separated by filtration. The recovered catalyst was washed with 3 ml of acetone to remove any organic residues present in the catalyst. The recovered catalyst was dried at 100 °C and subjected to the consecutive runs with same experimental condition. The yield of the product and the reaction time were not much affected up to fifth run after that a slight decrement in the yield of the product was observed. From these results, it has been concluded that 10 mol % of SnO2 NPs can be efficiently reused for more than five to six consecutive runs without loss in its catalytic activity (Fig. 5). After the sixth run, the recovered SnO2 NPs were further subjected to FE-SEM analysis which was shown in Fig. 6.
Conclusion
We have demonstrated the SnO2 NPs as an effective catalyst for the synthesis of 2H-indazolo[2,1-b]phthalazine-triones derivatives via one-pot three-component condensation reaction of the aromatic aldehydes, dimedone/1,3-cyclohexanedione and phthalhydrazide under solvent-free condition at 80 °C. The SnO2 NPs have remarkable advantages such as cheap and efficient catalyst. The excellent yield of the product without using column chromatography, shorter reaction time and easy work-up procedure and the reusability of the catalyst are the added advantages of this method. The present protocol seems to be a better alternative compared to other reported protocols.
References
Taj, T., Kamble, R.R., Gireesh, T.M., Hunnur, R.K., Margankop, S.B.: One-pot synthesis of pyrazoline derivatised carbazoles as antitubercular, anticancer agents, their DNA cleavage and antioxidant activities. Eur. J. Med. Chem. 46, 4366–4373 (2011)
Bergstrom, F.W.: Heterocyclic nitrogen compounds. Chem. Rev. 35, 77–277 (1944)
Padmaja, A., Payani, T., Dinneswara Reddy, G., Padmavathi, V.: Synthesis, antimicrobial and antioxidant activities of substituted pyrazoles, isoxazoles, pyrimidine and thioxopyrimidine derivatives. Eur. J. Med. Chem. 44, 4557–4566 (2009)
Litvinov, V.P.: Multicomponent cascade heterocyclisation as a promising route to targeted synthesis of polyfunctional pyridines. Russ. Chem. Rev. 72, 69–85 (2003)
Xu, Y., Guo, Q.-X.: Syntheses of heterocyclic compounds under microwave irradiation. Heterocycles 63, 903–974 (2004)
Clark, M.P., Laughlin, S.K., Laufersweiler, M.J., Bookland, R.G., Brugel, T.A., Golebiowski, A., Sabat, M.P., Townes, J.A., VanRens, J.C., Djung, J.F., Natchus, M.G., De, B., Hsieh, L.C., Xu, S.C., Walter, R.L., Mekel, M.J., Heitmeyer, S.A., Brown, K.K., Juergens, K., Taiwo, Y.O., Janusz, M.J.: Development of orally bioavailable bicyclic pyrazolones as inhibitors of tumor necrosis factor-α production. J. Med. Chem. 47, 2724–2727 (2004)
Zhang, L., Guan, L.P., Sun, X.Y., Wei, C.X., Chai, K.Y., Quan, Z.S.: Synthesis and anticonvulsant activity of 6-alkoxy-[1, 2, 4]triazolo[3,4-a]phthalazines. Chem. Bio. Drug Des. 73, 313–319 (2009)
Nomoto, Y., Obase, H., Takai, H., Teranishi, M., Nakamura, J., Kubo, K.: Studies on cardiotonic agents. III.: synthesis of 1-[1-(6,7-dimethoxy-4-quinazolinyl)-4-piperidinyl]-3-substituted 2-imidazolidinone and 2-imidazolidinethione derivatives. Chem. Pharm. Bull. 38, 2467–2471 (1990)
Del Olmo, E., Barboza, B., Ybarra, M.I., Lopez-Perez, J.L., Carron, R., Sevilla, M.A., Bosellid, C., San Felicianoa, A.: Vasorelaxant activity of phthalazinones and related compounds. Bioorg. Med. Chem. Lett. 16, 2786–2790 (2006)
Barbuceanu, S.-F., Saramet, G., Almajan, G.L., Draghici, C., Barbuceanu, F., Bancescu, G.: New heterocyclic compounds from 1,2,4-triazole and 1,3,4-thiadiazole class bearing diphenylsulfone moieties. Synthesis, characterization and antimicrobial activity evaluation. Eur. J. Med. Chem. 49, 417–423 (2012)
Ryu, C.K., Park, R.E., Ma, M.Y., Nho, J.H.: Synthesis and antifungal activity of 6-arylamino-phthalazine-5,8-diones and 6,7-bis(arylthio)-phthalazine-5,8-diones. Bioorg. Med. Chem. Lett. 17, 2577–2580 (2007)
De, P., Baltas, M., Lamoral-Theys, D., Bruyere, C., Kiss, R., Bedos-Belval, F., Saffon, N.: Synthesis and anticancer activity evaluation of 2(4-alkoxyphenyl)cyclopropylhydrazides and triazolophthalazines. Bioorg. Med. Chem. 18, 2537–2548 (2010)
Abdalla, M.S.M., Hegab, M.I., Abo Taleb, N.A., Hasabelnaby, S.M., Goudah, A.: Synthesis and anti-inflammatory evaluation of some condensed [4-(3,4-dimethylphenyl)-1(2H)-oxo-phthalazin-2-yl]acetic acid hydrazide. Eur. J. Med. Chem. 45, 1267–1277 (2010)
Cheng, Y., Ma, B., Wudl, F.: Synthesis and optical properties of a series of pyrrolopyridazine derivatives: deep blue organic luminophors for electroluminescent devices. J. Mater. Chem. 9, 2183–2188 (1999)
Raghuvanshi, D.S., Singh, K.N.: A highly efficient green synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives and their photophysical studies. Tetrahedron Lett. 52, 5702–5705 (2011)
Atashkar, B., Rostami, A., Gholami, H., Tahmasbi, B.: Magnetic nanoparticles Fe3O4-supported guanidine as an efficient nanocatalyst for the synthesis of 2H-indazolo[2,1-b]phthalazine-triones under solvent-free conditions. Res. Chem. Intermed. 41, 3675–3681 (2015)
Kiasat, A.R., Noorizadeh, S., Ghahremani, M., Saghanejad, S.J.: Experimental and theoretical study on one-pot, three-component route to 2H-indazolo[2,1-b]phthalazine-triones catalyzed by nano-alumina sulforic acid. J. Mol. Struct. 1036, 216–225 (2013)
Sayyafi, M., Soorki, A.A., Bazgir, A.: One-pot synthesis and antibacterial activities of novel 1H-pyridazino[1,2-a]indazole-1,6,9(2H,11H)-triones. Chem. Pharm. Bull. 56, 1289–1291 (2008)
Varghese, A., Nizam, A., Kulkarni, R., George, L.: Solvent-free synthesis of 2H-indazolo[2,1-b] phthalazine-triones promoted by cavitational phenomenon using iodine as catalyst. Eur. J. Chem. 4, 132–137 (2013)
Shaterian, H.R., Hosseinian, A., Ghashang, M.: Reusable silica supported poly phosphoric acid catalyzed three-component synthesis of 2H-indazolo [2, 1-b] phthalazine-trione derivatives. Arkivoc II, 59–67 (2009)
Pouramiri, B., TavakolinejadKermani, E.: One-pot, four-component synthesis of new 3,4,7,8-tetrahydro-3,3-dimethyl-11-aryl-2H-pyridazino[1,2-a]indazole-1,6,9(11H) triones and 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-triones using an acidic ionic liquid N, N-diethyl-N-sulfoethanammonium chloride ([Et3 N–SO3H]Cl) as a highly efficient and recyclable catalyst. Tetrahedron Lett. 57, 1006–1010 (2016)
Hasaninejad, A., Zare, A., Shekouhy, M.: Highly efficient synthesis of triazolo [1,2-a] indazole-triones and novel spirotriazolo [1,2-a] indazole-tetraones under solvent-free conditions. Tetrahedron 67, 390–400 (2011)
AlinasabAmiri, A., Javanshir, S., Dolatkhah, Z., Dekamin, M.G.: SO3H-functionalized mesoporous silica materials as solid acid catalyst for facile and solvent-free synthesis of 2H-indazolo[2,1-b]phthalazine-1,6,11-trione derivatives. New J. Chem. 39, 9665–9671 (2015)
Hassankhani, A., Mosaddegh, E., Yousef Ebrahimipour, S.: Tungstosilicic acid as an efficient catalyst for the one-pot multicomponent synthesis of triazolo[1,2-a]indazole-1,3,8-trione derivatives under solvent-free conditions. Arab. J. Chem. 9, S936–S939 (2016)
Tavakoli, H.R., Moosavi, S.M., Bazgir, A.: ZrOCl2·8H2O as an efficient catalyst for the three-component synthesis of triazoloindazoles and indazolophthalazines. J. Korean Chem. Soc. 57, 472–475 (2013)
Davarpanah, J., Rezaee, P., Elahi, S.: Synthesis and characterization of a porous acidic catalyst functionalized with an imidazole ionic liquid, and its use for synthesis of phthalazinedione and phthalazinetrione heterocyclic compounds. Res. Chem. Intermed. 41, 9903–9915 (2015)
Nagarapu, L., Bantu, R., Mereyala, H.B.: TMSCl-mediated one-pot, three-Component synthesis of 2H-indazolo[2,1-b]phthalazine-triones. J. Heterocyclic Chem. 46, 728–731 (2009)
Khurana, J.M., Magoo, D.: Efficient one-pot syntheses of 2H-indazolo[2,1-b] phthalazine-triones by catalytic H2SO4 in water–ethanol or ionic liquid. Tetrahedron Lett. 50, 7300–7303 (2009)
Toure, B.B., Hall, D.G.: Natural product Synthesis using multicomponent reaction strategies. Chem. Rev. 109, 4439–4486 (2009)
Razvan, C.C., Ruijter, E., Orru, R.V.A.: Multicomponent reactions: advanced tools for sustainable organic synthesis. Green Chem. 16, 2958–2975 (2014)
Bamoniri, A., Moshtael-Arani, N.: Nano-Fe3O4 encapsulated-silica supported boron trifluoride as a novel heterogeneous solid acid for solvent-free synthesis of arylazo-1-naphthol derivatives. RSC Adv. 5, 16911–16920 (2015)
Maddila, S.N., Maddila, S., vanZyl, W.E., Jonnalagadda, S.B.: Mn doped ZrO2 as a green, efficient and reusable heterogeneous catalyst for the multicomponent synthesis of pyrano[2,3-d]-pyrimidine derivatives. RSC Adv. 5, 37360–37366 (2015)
Tarannum, S., Siddiqui, Z.N.: Fe(OTs)3/SiO2: a novel catalyst for the multicomponent synthesis of dibenzodiazepines under solvent-free conditions. RSC Adv. 5, 74242–74250 (2015)
Karthikeyan, G., Pandurangan, A.: Heteropolyacid (H3PW12O40) supported MCM-41: an efficient solid acid catalyst for the green synthesis of xanthenedione derivatives. J. Mol. Catal. A-Chem. 311, 36–45 (2009)
Adharvana Chari, M., Syamasundar, K.: Silicagel supported sodium hydrogensulfate as a heterogenous catalyst for high yield synthesis of 3, 4-dihydropyrimidin-2 (1H)-ones. J. Mol. Catal. A-Chem. 221, 137–139 (2004)
Lu, Y.C., Ma, C., Alvarado, J., Kidera, T., Dimov, N., Meng, Y.S., Okada, S.: Electrochemical properties of tin oxide anodes for sodium-ion batteries. J. Power Sources 284, 287–295 (2015)
Bayal, N., Jeevanandam, P.: Sol-gel synthesis of SnO2-MgO nanoparticles and their photocatalytic activity towards methylene blue degradation. Mater. Res. Bull. 48, 3790–3799 (2013)
Sharp, S.L., Kumar, G., Vicenzi, E.P., Bocarsly, A.B., Heibel, M.: Formation and structure of a tin-iron oxide solid-state system with potential applications in carbon monoxide sensing through the use of cyanogelchemistry. Chem. Mater. 10, 880–885 (1998)
Yang, G., Haibo, Z., Biying, Z.: Monolayer dispersion of oxide additives on SnO2 and their promoting effects on thermal stability of SnO2 ultrafine particles. J. Mater. Sci. 35, 917–923 (2000)
Chopra, K.L., Major, S., Pandya, D.K.: Transparent conductors-A status review. Thin Solid Flims 102, 1–46 (1983)
Ferrere, S., Zaban, A., Gregg, B.A.: Dye sensitization of nanocrystalline tin oxide by perylene derivatives. J. Phys. Chem. B. 101, 4490–4493 (1997)
Sharghi, H., Ebrahimpourmoghaddam, S., Memarzadeh, R., Javadpour, S.: Tin oxide nanoparticles (NP-SnO2): preparation, characterization and their catalytic application in the Knoevenagel condensation. J. Iran. Chem. Soc. 10, 141–149 (2013)
Fallah, N.S., Mokhtary, M.: Tin oxide nanoparticles (SnO2-NPs): an efficient catalyst for the one-pot synthesis of highly substituted imidazole derivatives. J. Taibah Univ. Sci. 9, 531–537 (2015)
Dehbashi, M., Aliahmad, M., Shafiee, M.R.M., Ghashang, M.: SnO2 nanoparticles: preparation and evaluation of their catalytic activity in the oxidation of aldehyde derivatives to their carboxylic acid and sulfides to sulfoxide analogs. Phosphorus Sulfur 188, 864–872 (2013)
Yelwande, A.A., Navgire, M.E., Tayde, D.T., Arbad, B.R., Lande, M.K.: SnO2/SiO2 nanocomposite catalyzed one-pot synthesis of 2-arylbenzothiazole derivatives. Bull. Korean Chem. Soc. 33, 1856–1860 (2012)
Zolfigol, M.A., Baghery, S., Moosavi-Zare, A.R., Vahdat, S.M., Alinezhad, H., Norouzi, M.: Design of 1-methylimidazolium tricyanomethanide as the first nanostructured molten salt and its catalytic application in the condensation reaction of various aromatic aldehydes, amides and β-naphthol compared with tin dioxide nanoparticles. RSC Adv. 5, 45027–45037 (2015)
Lopez-Macipe, A., Gomez-Morales, J., Rodriguez-Clemente, R.: Nanosized hydroxyapatite precipitation from homogeneous calcium/citrate/phosphate solutions using microwave and conventional heating. Adv. Mater. 10, 49–53 (2009)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Maheswari, C.S., Shanmugapriya, C., Revathy, K. et al. SnO2 nanoparticles as an efficient heterogeneous catalyst for the synthesis of 2H-indazolo[2,1-b]phthalazine-triones. J Nanostruct Chem 7, 283–291 (2017). https://doi.org/10.1007/s40097-017-0238-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-017-0238-1