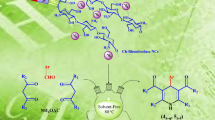
Abstract
1,4-Dihydropyridines are synthesized from various aromatic aldehyde, dimedone or 1,3-cyclohexandione, ethyl acetoacetate or methyl acetoacetate and ammonium acetate catalyzed by magnetite/chitosan at room temperature under mild reaction conditions. This process offers an easy and efficient synthesis of 1,4-dihydropyridine derivatives in high yields. The benefits of this protocol are simplicity, nontoxicity, low cost, simple work-up, and an environmentally benign nature. The catalyst is recovered by filtration and reused at least five times without significant loss in catalytic activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, applying heterogeneous catalysts in organic synthesis is become popular because they are removable from the reaction media by simple filtration and reusable. In addition, to achieve high activity in heterogeneous catalysts, composite nanoparticles are the logical strategy because of a large surface to volume ratio, thus increasing catalytic activity. Furthermore, magnetic nanoparticles, especially supported magnetic nanocatalysts, have attracted considerable interest in both academic and industrial researches because they are viable alternatives to conventional materials, readily available, simple separation by an external magnet and high degree of chemical stability in various organic and inorganic solvents [1–10].
Dihydropyridines are privileged heterocyclic ring systems because of their broad and significant pharmacological properties and they are also analogues of NADH coenzymes. 1,4-Dihydropyridine derivatives have applications as calcium channel blockers for the treatment of cardiovascular diseases including hypertension. They are also used as antidiabetic agents, anti-tumor, geroprotective, anti-therosclerotic, and bronchodilator [11–14]. The first molecules of 1,4-dihydropyridines were reported by Hantzch in 1882. It is a one-pot four component synthesis of 1,4-dihydropyridine by two molecules of ethyl acetoacetate, aldehyde and ammonia. This procedure does not need any additives and the reaction was done either at reflux in alcohol or in acetic acid; furthermore, the reaction suffer from long reaction times and low-yields of products [15]. Due to significance of dihydropyridine derivatives in the synthesis of various class of drugs several synthesis procedures have been presented such as use of microwaves [16, 17], high temperature at reflux [18–22], ionic liquid [23], SiO2/HClO4 [24], SiO2/NaHSO4 [25], I2 [26], Bakers’ yeast [27], metal triflates [28], tetrabutylammonium hydrogen sulfate [29], organocatalysts [30], PTSA [31], iron trifluoroacetate [32], TMSCl [33] and Ni nanoparticles [34].
Some of the above-mentioned methods for the synthesis of 1,4-dihydropyridine derivatives have one or more negative points such as expensive reagents, tedious work-up, moisture sensitive, toxic and harsh reaction conditions, thus developing an efficient protocol with a powerful catalyst for the synthesis of 1,4-dihydropyridine is still of prime importance. On the other hand, catalysis is a key part of green chemistry, and one of the fundamental needed challenges facing chemists now is to design and apply of eco-friendly catalysts [35–38]. A stable and green catalyst is defined as low preparation cost, high activity, great selectivity, high stability, efficient recovery, and good recyclability [39].
In continuation of our recent works to applying heterogeneous nanocatalysts in organic synthesis [40–44], herein, we wish to report Fe3O4@chitosan as a green heterogeneous catalyst for the synthesis of 1,4-dihydropyridine derivatives 4a–4ai via a one-pot four-component Hantzsch condensation reaction using various aromatic aldehyde (1) 5,5-dimethyl-1,3-cyclohexanedione (dimedone) or 1,3-cyclohexandione (2) ethyl acetoacetate or methyl acetoacetate (3) and ammonium acetate in ethanol in good to excellent yields at room temperature (Scheme 1). The main advantages of this protocol are using ethanol as a green solvent, inexpensive catalyst and easy preparation, mild reaction conditions, high yields, easy work-up, simple filtration and ability of reusing catalyst.
Experimental
General
All solvents, chemicals and reagents were purchased from Merck, Fluka and Aldrich. Melting points were measured on an Electrothermal 9100 apparatus and are uncorrected. The scanning electron microscopy (SEM) images were obtained on a Seron AIS 2100. Fourier transform infrared (FT–IR) spectra were recorded using Perkin-Elmer spectrometer; using pellets of the nanomaterials diluted with KBr. The 1H NMR spectra were recorded on Bruker DRX-300 Avance spectrometer at 300.13 MHz. The elemental analyses were performed with an Elementar Analysensysteme GmbH VarioEL.
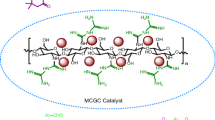
Preparation of Fe3O4@chitosan nanocomposite
First, 1 g of chitosan powder was added into a 100 mL of 0.1 M acetic acid and the mixture was stirred to form a 1 % (w/v) clear chitosan solution. Then, the homogeneous solution was filtered through a synthetic cloth to remove any undissolved materials and degassed by keeping the solution into vacuum oven for 3 h to remove the trapped air bubbles. After that, 2 mL of glycolic acid and calculated amount of Fe3O4 nanoparticles [black powder, average particle size 30 nm (TEM), purity 99 % and specific surface area 55 m2/g] were dispersed in the chitosan solution by stirring at room temperature and then it was sonicated for 1 h. The resulting solution was stirred overnight at room temperature. After that, the resulted gel separated from the reaction mixture by a permanent magnet, washed several times with EtOH, and dried on a ceramic plate at room temperature during 48 h to obtain the chitosan-supported magnetite nanoparticles.
Synthesis of dihydropyridine derivatives
A mixture of an aldehyde (1.0 mmol), dimedone or 1,3-cyclohexanedione (1.0 mmol), ethyl acetoacetate or methyl acetoacetate (1.0 mmol) and ammonium acetate (1.0 mmol) in 5 mL of EtOH was stirred at room temperature in the presence of a catalytic amount of Fe3O4@chitosan (0.03 g) for the appropriate times to obtain dihydropyridines derivatives. After completion of the reaction, as indicated by thin layer chromatography (TLC) (n-hexane/ethyl acetate 3/1), the catalyst was separated magnetically from the reaction mixture, washed with ethanol, and used for subsequent reactions after drying. Pure dihydropyridines 4a–4ai was afforded by evaporation of the solvent, followed by recrystallization from EtOH.
Spectral data of the product 4ai
IR (KBr) (υmax cm−1) = 3,274, 3,207, 2,960, 1,701, 1,647, 1,604, 1,492, 1,379, 1,215, 1,072. 1H NMR (300 MHz, CDCl3): δH (ppm) = 1.04 (s, 3H, CH3), 1.10 (s, 3H, CH3), 1.27 (t, 3H, J = 7.1 Hz, CH3), 2.22–2.34 (m, 4H, 2CH2), 2.38 (s, 3H, CH3), 4.16 (q, 2H, J = 7.1 Hz, OCH2), 5.42 (s, 1H, CH), 6.19 (br s, 1H, NH), 6.84 (d, 2H, J = 1.8 Hz, H–Ar), 7.03 (t, 1H, J = 2.1 Hz, H–Ar). Anal. Calcd for C19H23NO3S: C, 66.06; H, 6.71; N, 4.05. Found: C, 67.11; H, 6.63; N, 3.94.
Results and discussion
The Fe3O4@chitosan nanocatalyst was first prepared by a sol–gel method modified in our previous reports [42]. Then, it was characterized by SEM analysis. The particle size was studied by SEM and the identification of Fe3O4@chitosan morphology was based on the analysis of SEM images. The obtained SEM images of nanoparticles clearly showed that Fe3O4 nanoparticles were properly supported on chitosan.
To optimize the reaction conditions, we checked the four-component condensation reaction of dimedone (1 mmol), ethyl acetoacetate (1 mmol), 4-chlorobenzaldehyde (1 mmol) and ammonium acetate (1 mmol) in the presence of different catalytic amounts of Fe3O4@chitosan at room temperature in 5 mL of EtOH, as a model reaction. It was found that 0.03 g of catalyst was enough to catalyze the reaction to produce high yields of dihydropyridines derivatives. As shown in Table 1 (Entries 1–4), using 0.03 g of the catalyst was enough to progress the reaction and an increment of the catalyst amount did not improve the yields. In the second stage the effect of solvent was studied. As can be seen from Table 1 (Entries 5–9), it was found that EtOH is the best solvent for this reaction to produce high yields in short reaction time in comparison with other polar, non-polar, protic and aprotic solvents.
To study the generality of this method, different types of starting material were reacted in the synthesis of 1,4-dihydropyridines. As illustrated in Table 2, aromatic aldehyde with both electron withdrawing groups and electron donating groups react well to give the products in good to excellent yields. As it predicted starting from aldehyde with electron withdrawing groups (such as nitro group) the condensation reaction occurs in the lower time in comparison with electron donating groups (such as alkoxyl group).
As shown in Scheme 2, the proposed mechanism of this reaction could be in two forms. In step 2 and 2′ which are Knoevenagel reaction involves coupling of aldehyde with active methylene compound. Furthermore, in step 3 and 3′ a Michael addition of intermediates gives the target products.
In addition, the reusability of this heterogeneous nanocatalyst is one of its important plus point and also this potency leads us to use it in commercial and industrial applications. One of the natural ability of this heterogeneous catalyst that makes it special is easy separation from the reaction media by an external magnetic bar. By washing with acetone or ethanol and drying it at room temperature it can be reused several times. As indicated in Table 3, the reusability of the present composite nanocatalyst is examined in the model reaction.
Conclusion
In summary, Fe3O4@chitosan was prepared and used as a green, magnetically recyclable and efficient composite nanocatalyst for the synthesis of chemically and biologically important dihydropyridine derivatives by a simple, clean, eco-friendly and inexpensive method. This protocol can be applied in large-scale and industrial synthesis of heterocyclic compounds, because it can be simple recovered and reused several times without significant decreasing in its catalytic activity.
Referenc es
Gawande, M.B., Luque, R., Zboril, R.: The rise of magnetically recyclable nanocatalysts. Chem. Soc. Rev. 42, 3371–3393 (2013)
Gawande, M.B., Brancoa, P.S., Varma, R.S.: Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem. Soc. Rev. 42, 3371–3393 (2013)
Wang, D., Astruc, D.: Fast-growing field of magnetically recyclable nanocatalysts. Chem. Rev. 114, 6949–6985 (2014)
Kainz, Q.M., Linhardt, R., Grass, R.N.: Palladium nanoparticles supported on magnetic carbon-coated cobalt nanobeads: highly active and recyclable catalysts for alkene hydrogenation. Adv. Funct. Mater. 24, 2020–2027 (2014)
Shelke, S.N., Bankar, S.R., Mhaske, G.R., Kadam, S.S., Murade, D.K.: Iron oxide-supported copper oxide nanoparticles (Nanocat-Fe-CuO): magnetically recyclable catalysts for the synthesis of pyrazole derivatives, 4-methoxyaniline, and Ullmann-type condensation reactions. ACS Sustain. Catal. Eng. 2, 1699–1706 (2014)
Sá, S., Gawande, M.B., Velhinho, A., Veiga, J.P., Bundaleski, N.: Magnetically recyclable magnetite–palladium (Nanocat-Fe–Pd) nanocatalyst for the Buchwald-Hartwig reaction. Green Chem. 16, 3494–3500 (2014)
Gawande, M.B., Rathi, I., Nogueira, D.: Magnetite-supported sulfonic acid: a retrievable nanocatalyst for the Ritter reaction and multicomponent reactions. Green Chem. 15, 1895–1899 (2013)
Shokouhimehr, M., Piao, Y., Kim, J., Jang, Y., Hyeon, T.: A magnetically recyclable nanocomposite catalyst for olefin epoxidation. Angew. Chem. Int. Ed. 46, 7039–7043 (2007)
Shirini, F., Abedini, M.: Application of nanocatalysts in multi-component reactions. J. Nanosci. Nanotechnol. 13, 4838–4860 (2013)
Montazeri, H., Amani, A., Shahverdi, H.R., Haratifar, E., Shahverdi, A.R.: Separation of the defect-free Fe3O4–Au core/shell fraction from magnetite-gold composite nanoparticles by an acid wash treatment. J. Nanostruct Chem. 3, 25–28 (2013)
Sausins, A., Duburs, G.: Synthesis of 1,4-dihydropyridines by cyclocondensation reactions. Heterocycles 27, 269–289 (1988)
Gaudio, A.C., Korokovas, A., Takahata, Y.: Quantitative structure-activity relationships for 1,4-dihydropyridine calcium channel antagonists (nifedipine analogues): a quantum chemical/classical approach. J. Pharm. Sci. 83, 1110–1115 (1994)
Mager, P.P., Coburn, R.A., Solo, A.J., Triggle, D.J., Rothe, H.: QSAR, diagnostic statistics and molecular modelling of 1,4-dihydropyridine calcium antagonists: a difficult road ahead. Drug Des. Disc. 8, 273–289 (1992)
Gordeev, M.F., Patel, D.V., Gordon, E.M.: Approaches to combinatorial synthesis of heterocycles: a solid-phase synthesis of 1,4-dihydropyridines. J. Org. Chem. 61, 924–982 (1996)
Hantzsch, A.: Ueber die synthese pyridinartiger verbindungenaus acetessigäther und aldehyd ammoniak. Justus Liebigs Ann. Chem. 215, 1–82 (1882)
Agarwal, A., Chauhan, P.M.S.: Solid supported synthesis of structurally diverse dihydropyrido[2,3-d]pyrimidines using microwave irradiation. Tetrahedron Lett. 46, 1345–1348 (2005)
Ohberg, L., Westman, J.: An efficient and fast procedure for the Hantzsch dihydropyridine synthesis under microwave conditions. Synlett 8, 1296–1298 (2001)
Breitenbucher, J.G., Figliozzi, G.: Solid-phase synthesis of 4-aryl-1,4-dihydropyridines via the Hantzsch three component condensation. Tetrahedron Lett. 41, 4311–4315 (2000)
Liang, J.C., Yeh, J.L., Wang, C.S., Liou, S.F., Tasi, C.H., Chen, I.: Heterocyclic bibenzimidazole derivatives as topoisomerase I inhibitors. J. Bioorg. Med. Chem. Lett. 10, 719–723 (2002)
Miri, R., Niknahad, H., Vesal, G., Shafiee, A.: Synthesis and calcium channel antagonist activities of 3-nitrooxyalkyl, 5-alkyl 1,4-dihydro-2,6-dimethyl-4-(1-methyl-5-nitro-2-imidazolyl)-3,5-pyridinedicarboxylates. IL Farmaco 57, 123–128 (2002)
Dondoni, A., Massi, A., Minghini, E., Sabbatini, S., Bertoasi, V.: Model studies toward the synthesis of dihydropyrimidinyl and pyridyl α-amino acids via three-component biginelli and Hantzsch cyclocondensations. J. Org. Chem. 68, 6172–6183 (2003)
Dondoni, A., Massi, A., Minghini, E., Bertoasi, V.: Multicomponent Hantzsch cyclocondensation as a route to highly functionalized 2- and 4-dihydropyridylalanines, 2- and 4-pyridylalanines, and their n-oxides: preparation via a polymer-assisted solution-phase approach. Tetrahedron 60, 2311–2326 (2004)
Ji, S.J., Jiang, Z.Q., Lu, J., Loh, T.P.: Facile ionic liquids-promoted one-pot synthesis of polyhydroquinoline derivatives under solvent free conditions. Synlett 5, 831–832 (2004)
Maheswara, M., Siddaiah, V., Damu, G.L., VenkataRao, C.: An efficient one-pot synthesis of polyhydroquinoline derivatives via Hantzsch condensation using heterogeneous catalyst under solvent-free conditions. Arkivoc 2, 201–206 (2006)
Chari, M.A., Syamasundar, K.: Silica gel/NaHSO4 catalyzed one-pot synthesis of Hantzsch 1,4-dihydropyridines at ambient temperature. Catal. Commun. 6, 624–626 (2005)
Ko, S., Sastry, M.N.V., Lin, C., Yao, C.F.: Molecular iodine-catalyzed one-pot synthesis of 4-substituted-1,4-dihydropyridine derivatives via Hantzsch reaction. Tetrahedron Lett. 46, 5771 (2005)
Kumar, A., Maurya, R.A.: Bakers’ yeast catalyzed synthesis of polyhydroquinoline derivatives via an unsymmetrical Hantzsch reaction. Tetrahedron Lett. 48, 3887–3890 (2007)
Wang, L.M., Sheng, J., Zhang, L., Han, J.W., Fan, Z., Tian, H., Qian, C.T.: Facile Yb(OTf)3 promoted one-pot synthesis of polyhydroquinoline derivatives through Hantzsch reaction. Tetrahedron 61, 1539–1543 (2005)
Tewari, N., Dwivedi, N., Tripathi, R.P.: Tetrabutylammonium hydrogen sulfate catalyzed eco-friendly and efficient synthesis of glycosyl 1,4-dihydropyridines. Tetrahedron Lett. 45, 9011–9014 (2004)
Kumar, A., Maurya, R.A.: Synthesis of polyhydroquinoline derivatives through unsymmetric Hantzsch reaction using organocatalysts. Tetrahedron 63, 1946–1952 (2007)
Cherkupally, S.R., Mekalan, R.: PTSA catalyzed facile and efficient synthesis of polyhydroquinoline derivatives through Hantzsch multi-component condensation. Chem. Pharm. Bull. 56, 1002–1004 (2008)
Adibi, H., Samimi, H.A., Beygzadeh, M.: Iron(III) trifluoroacetate and trifluorometh-anesulfonate: recyclable Lewis acid catalysts for one-pot synthesis of 3,4-dihydropyrimidinones or their sulfur analogues and 1,4-dihydropyridines via solvent-free Biginelli and Hantzsch condensation protocols. Catal. Commun. 8, 2119–2124 (2007)
Sabitha, G., Reddy, G.S.K., Reddy, C.S., Yadav, J.S.: A novel TMSI-mediated synthesis of Hantzsch 1,4-dihydropyridines at ambient temperature. Tetrahedron Lett. 44, 4129–4131 (2003)
Sapkal, S.B., Shelke, K.F., Shingate, B.B., Shingare, M.S.: Nickel nanoparticle-catalyzed facile and efficient one-pot synthesis of polyhydroquinoline derivatives via Hantzsch condensation under solvent-free conditions. Tetrahedron Lett. 50, 1754–1756 (2009)
Sheldon, R.A.: Fundamentals of green chemistry: efficiency in reaction design. Chem. Soc. Rev. 41, 1437–1451 (2012)
Walsh, P.T., Li, H., De Parrodi, C.A.: A green chemistry approach to asymmetric catalysis: solvent-free and highly concentrated reactions. Chem. Rev. 107, 2503–2545 (2007)
NasirBaig, R.B., Varma, R.S.: Stereo- and regio-selective one-pot synthesis of triazole-based unnatural amino acids and β-amino triazoles. Chem. Commun. 48, 5853–5855 (2012)
NasirBaig, R.B., Varma, R.S.: Alternative energy input: mechanochemical, microwave and ultrasound-assisted organic synthesis. Chem. Soc. Rev. 41, 1559–1584 (2012)
Gawande, S.B., Brancoa, P.S., Varma, R.S.: Nanocatalysis and prospects of green chemistry. Chem. Sus. Chem. 5, 65–68 (2012)
Maleki, A.: Fe3O4/SiO2 nanoparticles: an efficient and magnetically recoverable nanocatalyst for the one-pot multicomponent synthesis of diazepines. Tetrahedron 68, 7827–7829 (2012)
Maleki, A.: One-pot multicomponent synthesis of diazepine derivatives using terminal alkynes in the presence of silica-supported superparamagnetic iron oxide nanoparticles. Tetrahedron Lett. 54, 2055–2059 (2013)
Maleki, A., Ghamari, N., Kamalzare, M.: Chitosan-supported Fe3O4 nanoparticles: a magnetically recyclable heterogeneous nanocatalyst for the syntheses of multifunctional benzimidazoles and benzodiazepines. RSC Adv. 4, 9416–9423 (2014)
Maleki, A., Kamalzare, M.: Fe3O4@cellulose composite nanocatalyst: preparation, characterization and application in the synthesis of benzodiazepines. Catal. Commun. 53, 67–71 (2014)
Maleki, A., Rahimi, R., Maleki, S., Hamidi, N.: Synthesis and characterization of magnetic bromochromate hybrid nanomaterials with triphenylphosphine surface-modified iron oxide nanoparticles and their catalytic application in multicomponent reactions. RSC Adv. 4, 29765–29771 (2014)
Tajbakhsh, M., Alinezhad, H., Norouzi, M., Akbari, M., Baghery, S.: Proticpyridinium ionic liquid as a green and highly efficient catalyst for the synthesis of polyhydroquinoline derivatives via Hantzsch condensation in water. J. Mol. Liq. 177, 44–48 (2013)
Song, S.J.J., Shan, Z.X., Jin, Y.: One-pot synthesis of hexahydroquinolines via Hantzsch four-component reaction catalyzed by a cheap amino alcohol. Synth. Commun. 40, 3067–3077 (2010)
Zare, A., Abi, F., Moosavi-Zare, A.R., Beyzavi, M.H., Zolfigol, M.A.: Synthesis, characterization and application of ionic liquid 1,3-disulfonic acid imidazolium hydrogen sulfate as an efficient catalyst for the preparation of hexahydroquinolines. J. Mol. Liq. 178, 113–121 (2013)
Safari, J., Banitaba, S.H., Khalili, S.H.: Cobalt nanoparticles promoted highly efficient one pot four-component synthesis of 1,4-dihydropyridines under solvent-free conditions. Chin. J. Catal. 32, 1850–1855 (2011)
Heravi, M., Saeedi, M., Karimi, N., Zakeri, M., Beheshtiha, Y.S., Davoodnia, A.: Brønsted acid ionic liquid [(CH2)4SO3HMIM][HSO4] as novel catalyst for one-pot synthesis of Hantzsch polyhydroquinoline derivatives. Synth. Commun. 40, 523–529 (2010)
Singh, S.K., Singh, K.N.: Glycine-catalyzed easy and efficient one-pot synthesis of polyhydroquinolines through Hantzsch multicomponent condensation under controlled microwave. J. Heterocycl. Chem. 47, 194–198 (2010)
Surasani, R., Kalita, D., Rao, A.V.D., Yarbagi, K., Chandrasekhar, K.B.: FeF3 as a novel catalyst for the synthesis of polyhydroquinoline derivatives via unsymmetrical Hantzsch reaction. J. Fluor. Chem. 135, 91–96 (2012)
Peng, H.N., Zheng, D.G., Peng, X.M.: p-Toluene sulfonic acid catalyzed one-pot synthesis of unsymmetrical 1,4-dihydropyridines derivatives via Hantzsch reaction. Asian J. Chem. 23, 1833–1837 (2011)
Yang, X.H., Zhou, Y.H., Liu, C.H.G., Lin, X.Y., Cui, J.F., Zhang, P.H.: Synthesis and antioxidant evaluation of novel 4-aryl-hexahydroquinolines from lignin. Arkivoc 10, 327–329 (2011)
Hong, M., Cai, C.H., Yi, W.B.: Hafnium (IV) bis(perfluorooctanesulfonyl)imide complex catalyzed synthesis of polyhydroquinoline derivatives via unsymmetrical Hantzsch reaction in fluorous medium. J. Fluorine Chem. 131, 111–114 (2010)
Li, J., He, P., Yu, Ch.: DPTA-catalyzed one-pot regioselective synthesis of polysubstituted pyridines and 1,4-dihydropyridines. Tetrahedron 68, 4138–4144 (2012)
Mohammadi, A., Hadadzahmatkesh, A., Asghariganjeh, M.R.: FeNH4(SO4)2·12H2O (alum)-catalyzed preparation of 1,4-dihydropyridines: improved conditions for the Hantzsch reaction. Monatsh. Chem. 143, 931–933 (2012)
Qin, X.Y., Jin, T.S., Zhou, Z.X., Li, T.S.H.: Silica sulfuric acid: an efficient and versatile catalyst for one-pot synthesis of substituted 5-oxo-1,4,5,6,7,8-hexahydroquinoline derivatives. Asian J. Chem. 22, 1179–1182 (2010)
Nasr-Esfahani, M., Abdizadeh, T.: Vanadatesulfuric acid-catalyzed novel and eco-benign one-pot synthesis of polyhydroquinoline derivatives under solvent-free conditions. Orient. J. Chem. 28, 1249–1258 (2012)
Sainani, J.B., Shah, A.C., Arya, V.P.: Synthesis of 4-aryl-1,4,5,6,7,8-hexahydro-5-oxo- 2,7,7-trimehyl-quinoline-3-carboxylates and amides. Indian J. Chem. Sect. B 33, 526–531 (1994)
Acknowledgments
The authors gratefully acknowledge the partial support from the Research Council of the Iran University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Maleki, A., Kamalzare, M. & Aghaei, M. Efficient one-pot four-component synthesis of 1,4-dihydropyridines promoted by magnetite/chitosan as a magnetically recyclable heterogeneous nanocatalyst. J Nanostruct Chem 5, 95–105 (2015). https://doi.org/10.1007/s40097-014-0140-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-014-0140-z