Abstract
FeNH4(SO4)2·12H2O (alum) efficiently catalyzes the one-pot three-component reaction of dimedone, aldehydes, and 3-aminocrotonate to afford 1,4-dihydropyridines. The work-up is easy, and the products are obtained in good to excellent yields and high purity.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The conventional synthesis of 1,4-dihydropyridines (1,4-DHPs) introduced by Hantzsch in 1881 involves one-pot condensation of an aldehyde, ammonia, and a ketoester in acetic acid [1]. Several methods have been developed to improve the efficiency of the Hantzsch reaction for synthesis of 1,4-DHPs, for example the use of microwaves [2], ionic liquids [3], high temperature [4], and acid catalysts, for example Mg(ClO4) [5], CeCl3·7H2O/NaI [6], Sc(OTf)3 [7], HY-Zeolite [8], and p-TSA [9].
1,4-Dihydropyridines have been reported to have diverse biological activity, for example anticonvulsant, antianxiety, antitumor, antidiabetic, antidepressive, analgesic, vasodilator, sedative, hypnotic, and anti-inflammatory activity [10], in addition to acting as a cerebral anti-ischemic agent in the treatment of Alzheimer’s disease [11] and as a chemosensitizer in tumor therapy [12].
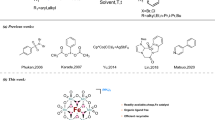
In continuation of our ongoing work on multi-component reactions (MCRs) [13–16], we now wish to report a facile and rapid one-pot three component reaction for preparation of DHP derivatives 4a–4k from dimedone 1, aldehydes 2a–2k, and 3-aminocrotonates 3a, 3b using non-toxic and readily available FeNH4(SO4)2·12H2O as heterogeneous catalyst (Scheme 1).
Results and discussion
When a mixture of dimedone 1, aldehyde 2a, and 3-aminocrotonate 3a in EtOH was stirred at r.t., the reaction was complete within 5 h. Work-up of the reaction mixture showed that dimethyl 1,4-dihydropyridine 4a was prepared in 94% yield (Scheme 1).
Encouraged by this success, we extended this reaction of dimedone to a range of other aldehydes 2b–2k and 3-aminocrotonates 3a, 3b under similar conditions, and 1,4-DHPs 4b–4k were obtained in good yield [21]. The optimized results are summarized in Table 1.
In summary, we have described a successful strategy, an efficient and convenient green synthesis, for the preparation of some new 1,4-DHPs in a three-component cyclocondensation reaction of dimedone, aldehydes, and 3-aminocrotonate. The method has several advantages, including high yield of products, use of inexpensive, nontoxic, and readily available FeNH4(SO4)2·12H2O catalyst, and an easy experimental work-up procedure, that make it a useful process for synthesis of 1,4-dihydropyridines.
Experimental
Melting points were measured in open capillary tubes on an Electrothermal 9200 apparatus. Mass spectra were recorded on a Shimadzu QP 1100 BX mass spectrometer. IR spectra were recorded as KBr pellets on a Shimadzu IR-470 spectrophotometer. 1H and 13C NMR spectra were determined on a Bruker 300 DRX Avance instrument at 300 and 75 MHz.
General procedure for preparation of 1,4-dihydropyridines 4a–4k
To a mixture of 1 mmol dimedone, 1 mmol aldehyde, and 1 mmol 3-aminocrotonate in 5 cm3 EtOH was added 0.1 g FeNH4(SO4)2·12H2O (20 mol%), and the reaction was stirred at r.t. for the time indicated in Table 1. After completion of the reaction (monitored by TLC, ethyl acetate–n-hexane, 1:1), water was added to the reaction mixture, and the resulting solid was separated by filtration. The crude product was recrystallized form ethanol.
The catalyst is very inexpensive and nontoxic. The catalyst in the aqueous phase can be recovered by removing the water under vacuum, then washing with acetone and ethanol and drying at room temperature. It can be recycled and used in subsequent reactions without reduction in activity.
Methyl 1,4,5,6,7,8-hexahydro-2,7,7-trimethyl-5-oxo-4-phenylquinoline-3-carboxylate (4h, C20H23NO3)
White powder; m.p.: 253–254 °C; IR (KBr): \( \overline{v} \) = 3,203, 3,016, 2,918, 1,707 (C=O), 1,626 (C=O) cm−1; 1H NMR (DMSO-d 6 ): δ = 0.95 (s, 3H, CH3), 1.14 (s, 3H, CH3), 2.13 (d, 1H, J = 12.7 Hz, CH2), 2.27 (d, 1H, J = 13.2 Hz, CH2), 2.32 (d, 1H, J = 15.2 Hz, CH2), 2.41 (s, 3H, CH3), 2.43 (d, 1H, J = 13.2 Hz, CH2), 3.64 (s, 3H, OCH3), 5.13 (s, 1H, CH), 7.12 (d, 1H, J = 7.2 Hz, Ar), 7.26 (t, 2H, J = 7.5 Hz, Ar), 7.32 (d, 2H, J = 7.5 Hz, Ar), 8.14 (s, 1H, NH) ppm; 13C NMR (DMSO-d 6 ): δ = 19.25, 27.36, 29.86, 32.85, 36.56, 40.17, 40.43, 40.72, 40.81, 41.03, 51.13, 105.21, 110.78, 126.15, 128.14, 128.19, 145.41, 147.68, 149.85, 168.43, 195.88 ppm; MS: m/z (%) = 325 (M+, 35), 248 (100), 77 (25), 51 (25), 39 (25).
Methyl 1,4,5,6,7,8-hexahydro-4-(3-methoxyphenyl)-2,7,7-trimethyl-5-oxoquinoline-3-carboxylate (4j, C21H25NO4)
White powder; m.p.: 258–259 °C; IR (KBr): \( \overline{v} \) = 3,203, 3,022, 2,888, 1,707 (C=O), 1,621 (C=O) cm−1; 1H NMR (DMSO-d 6 ): δ = 0.89 (s, 3H, CH3), 1.06 (s, 3H, CH3), 2.01 (d, 1H, J = 7.8 Hz, CH2), 2.07 (d, 1H, J = 15.5 Hz, CH2), 2.12 (d, 1H, J = 7.2 Hz, CH2), 2.29 (d, 1H, J = 8.5 Hz, CH2), 2.94 (s, 3H, CH3), 3.58 (s, 3H, OCH3), 3.70 (s, 3H, OCH3), 4.93 (s, 1H, CH), 6.85–7.02 (m, 4H, Ar), 8.98 (s, 1H, NH) ppm; 13C NMR (DMSO-d 6 ): δ = 19.18, 27.43, 29.93, 32.83, 35.65, 39.58, 39.85, 40.14, 40.42, 40.70, 40.98, 41.27, 51.10, 51.21, 55.40, 102.99, 109.91, 126.24, 126.44, 127.55, 130.31, 132.87, 146.38, 150.23, 150.32, 167.57, 194.86 ppm; MS: m/z (%) = 355 (M+, 65), 340 (25), 248 (100), 57 (40), 41 (30).
Methyl 1,4,5,6,7,8-hexahydro-2,7,7-trimethyl-4-(4-nitrophenyl)-5-oxoquinoline-3-carboxylate (4k, C20H22N2O5)
White powder; m.p.: 252–253 °C; IR (KBr): \( \overline{v} \) = 3,204, 3,016, 2,963, 1,712 (C=O), 1,612 (C=O) cm−1; 1H NMR (DMSO-d 6 ): δ = 1.03 (s, 3H, CH3), 1.23 (s, 3H, CH3), 2.11 (d, 1H, J = 9.4 Hz, CH2), 2.34 (d, 1H, J = 16.1 Hz, CH2), 2.50 (d, 1H, J = 14.2 Hz, CH2), 2.56 (s, 3H, CH3), 2.69 (d, 1H, J = 9.5 Hz, CH2), 3.74 (s, 3H, OCH3), 5.18 (s, 1H, CH), 7.62 (d, 2H, J = 8.4 Hz, Ar), 8.30 (d, 2H, J = 8.4 Hz, Ar), 9.51 (s, 1H, NH) ppm; 13C NMR (DMSO-d 6 ): δ = 19.24, 27.25, 29.87, 33.02, 37.28, 50.91, 51.68, 102.93, 109.91, 124.10, 129.47, 146.51, 147.31, 151.02, 155.67, 167.78, 195.21 ppm; MS: m/z (%) = 370 (M+, 60), 340 (25), 248 (100), 83 (40), 55 (80), 39 (85).
References
Gupta R, Gupta R, Paul S, Loupy A (2007) Synthesis 18:2835
Akbari JD, Tala SD, Dhaduk MF, Joshi HS (2008) Arkivoc xii:126
Sridhar R, Perumal PT (2005) Tetrahedron 61:2465
Dondoni A, Massi A, Minghini E, Sabbatini S, Bertoasi V (2003) J Org Chem 68:6172
Bartoli G, Krzysztof B, Bosco M, Carlone A, Galzerano P, Melchirro P, Sambri L (2007) Synlett 2897
Yadav LDS, Kapoor R (2008) Synlett 2348
Kikuchi S, Iwai M, Murayama H, Fukuzawa SI (2008) Tetrahedron Lett 49:114
Das B, Ravikanth B, Ramu R, Rao BV (2006) Chem Pharm Bull 54:1044
Cherkupally SR, Mekala R (2008) Chem Pharm Bull 56:1002
Mannhold R, Jablonka B, Voigdt W, Schoenafinger K, Schravan K (1992) Eur J Med Chem 27:229
Bretzel RG, Bollen CC, Maeser E, Federlin KF (1992) Drugs Futur 17:465
Boer R, Gekeler V (1995) Drugs Futur 20:499
Azizian J, Mohammadi AA, Karimi AR (2003) Synth Commun 33:415
Azizian J, Mohammadi AA, Karimi AR, Mohammadizadeh MR (2005) J Org Chem 70:350
Mohammadi AA, Akbarzadeh R, Rohi R (2009) Comb Chem High Throughput Screen 12:536
Azizian J, Mohammadi AA, Kohshari M, Karimi A, Mohammadizadeh MR (2007) J Heterocycl Chem 44:455
Kumar A, Maurya RA (2007) Tetrahedron 48:3887
Heravi MM, Bakhtiari K, Javadi NM, Bamoharram FF, Saeedi M, Oskooie HA (2007) J Mol Catal A Chem 264:50
Brietenbucher JG, Figliozzi G (2000) Tetrahedron Lett 41:4311
Kumar A, Maurya RA (2007) Tetrahedron 63:1946
See for mechanism: Li JJ (2002) Name reactions: a collection of detailed reaction mechanisms, Springer, Berlin
Acknowledgments
We gratefully acknowledge financial support from the Research Council of Islamic Azad University, Branch Sabzevar.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Mohammadi, A.A., Hadadzahmatkesh, A. & Asghariganjeh, M.R. FeNH4(SO4)2·12H2O (alum)-catalyzed preparation of 1,4-dihydropyridines: improved conditions for the Hantzsch reaction. Monatsh Chem 143, 931–933 (2012). https://doi.org/10.1007/s00706-011-0672-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-011-0672-6