Abstract
The application of transparent plastic bags with a gas barrier layer as inexpensive photobioreactors for H2 production by the cyanobacterium Anabaena sp. PCC 7120 ∆Hup mutant cells was explored outdoors in Moscow region for the first time. Two experiments were performed in July and August, the average daily temperature being 21.7 and 20.6 °C, the average daily light intensity being 290 and 340 µmol photon m−2 s−1 in experiment #1 and #2, respectively. The maximal H2 production rate was 20.6 mL day−1 L−1 of culture, with accumulation of 33.2 mL L−1 during 5 days and a final H2 content of 1.1% (v/v). Molecular nitrogen added to the Ar gas at 3.3% significantly affected neither the rate nor the duration of H2 production. Low morning temperatures as well as high daytime light intensities (especially at low cell concentrations) seemed to reduce the H2 production rate. The activities obtained were lower as compared to the previously reported data. It could be attributable to suboptimal weather conditions and simple device arrangement. However, results prove that H2 production by cyanobacteria is still feasible outdoors in plastic bags, the cheapest photobioreactors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photobiological production of hydrogen (H2) is considered to be one of the most promising technologies for replacing or complementing fossil fuel-derived energy. In contrast with electricity generated by solar panels and wind mills, H2 is more easily stored and transported from overseas [1,2,3,4,5].
Among phototrophic microorganisms, purple bacteria are characterized by the highest rates of H2 production. However, the presence of organic substances and anaerobic conditions are required for this process. Unlike that, autotrophic microorganisms such as nitrogen-fixing filamentous heterocyst-forming cyanobacteria do not require organic substances, have simple nutritional needs, use N2 from the air as the nitrogen source for growth and produce H2 using specialized cells called heterocysts.
Laboratory cultivation of cyanobacteria for biomass harvesting usually presents no difficulties, requiring only inorganic culture medium, air blowing with CO2 supplementation, gentle mixing and compliance with temperature and light regimes. A description of suitable photobioreactors (PhBR) for this purpose is presented in several reviews [6,7,8].
For large-scale production of cyanobacterial biomass, the simplest and cheapest solution is cultivation in open ponds or pools, but for harvesting H2, we need closed bioreactors [9]. The laboratory conditions for H2 production by cyanobacterial suspensions have been studied in detail. The main influencing factors are light intensity, temperature and the nitrogen source [10]. The highest rates of H2 production [100–130 μmol h−1 mg chlorophyll (Chl)−1] in short-term experiments were achieved using genetically modified strains (in particular, with elimination of the H2 uptake hydrogenase activity ∆Hup) [11, 12].
For laboratory-scale H2 production by cyanobacteria, one can use different types of PhBRs: cylindrical, panel, tubular, spiral with mechanical or airlift mixing [10, 13]. Experiments with Anabaena variabilis PCC 7120 and mutant AMC 414 (lacking the H2 uptake activity) were performed in a laboratory tubular PhBR with control of pH, temperature and optical density under chemostat and modes [14]. The production of H2 by ∆Hup mutants took place under air, unlike the wild types where in the presence of oxygen, H2 was used as the electron donor and its yield greatly decreased. In the Ar atmosphere, H2 evolution increased in both strains, but in the mutant it was maximal, reaching 13.8 mL L−1 h−1. Under simulated outdoor conditions in an automated helical tubular PhBR, A. variabilis PK84 did produce 230 mL H2 per PhBR (4.35 L) during 12-h light period under aeration [15].
Few attempts to obtain H2 outdoors using reactors with cyanobacteria have been made. The above-mentioned study of the A. variabilis PCC 7120 mutant (AMC 414) was also continued outdoors. In this case, the rate of H2 evolution in the chemostat mode was about 3 mL L−1 h−1 [14]. As another example, H2 production by A. variabilis PK84 in a spiral tubular PhBR during purging air supplemented with CO2 was observed in a batch or chemostat mode with pH control [16]. Under the experimental conditions (on the roof of a building in London), the average rate of H2 evolution was 8 mL L−1 h−1. Recently, Synechocystis sp. strain PCC 6803 was cultivated outdoors in 50-L tubular PhBR, with maximum H2 production rate of 0.05 mL L−1 h−1 under dark anaerobic conditions [17].
Although extensive researches have been carried out on photobiological H2 production by various photosynthetic organisms using various types of PhBRs, none of the systems are actually operating in a commercially viable state. For low-cost photobiological H2 production, one of the great issues is the need for inexpensive PhBRs. Amos [18] made a lifecycle assessment of H2 production by the green alga Chlamydomonas, and pointed out that the cost of PhBRs should not exceed US$ 10 m−2 for economically viable H2 production.
For inexpensive PhBRs, Sakurai et al. [1, 9, 19] proposed the use of large transparent plastic bags with low permeability to H2 floating on the sea surface on calm belts. The potential usefulness of such PhBRs was tested under laboratory conditions using the ∆Hup mutant of the cyanobacterium Nostoc sp. PCC 7422 [20]. However, the above experiments were carried on a small scale (the culture volume of 50 mL) under the light from fluorescent lamps in a laboratory.
To summarize, even if laboratory experiments suggest that cyanobacteria are promising for producing hydrogen, there are only a few outdoor studies. And no attempt has been made to make this process more cost effective.
The goal of our work was to explore the possibility of H2 production by Anabaena sp. PCC 7120 ∆Hup for the first time outdoors in Moscow region using cheap PhBRs—transparent plastic bags with a gas barrier layer (Be-P).
Materials and methods
The object of study and cultivation conditions
The strain used was a mutant strain of cyanobacteria Anabaena sp. PCC 7120 (ΔHup), created by disrupting the uptake hydrogenase gene hupL [21], and provided by Prof. Inoue, K., Kanagawa University, Japan.
The inoculum was grown photo-autotrophically in 300-mL Erlenmeyer flasks in Allen–Arnon medium [22] diluted 8 times (AA/8) supplemented with 5 mM TES-KOH buffer (pH 8.2), without adding bound nitrogen but with the addition of streptomycin and spectinomycin, 2 μg mL−1 each [21]. The cultivation was carried out at 27 °C in light (30 μmol photon m−2 s−1) under air with shaking at 95 rpm.
For experiments, culture (~ 14 L) was grown under the conditions indicated above (except shaking). A set of bottles (1.7 L) were sparged with air + 1% CO2 through sterile membrane filters with a pore size of 0.2 μm (Pall, Ann Arbor, MI, USA).
The design of PhBRs
To study H2 production, cyanobacteria were placed in PhBRs—36 cm × 50 cm flexible plastic Be-P bags [20], which have a polyacrylate gas barrier layer. In the first experiment, each PhBR was a flat bag with two ports: for culture and gas sampling [20]. In the second experiment, a rigid internal stainless steel frame was placed inside the bag, increasing the height of the bag to ensure the gas flow from one PhBR to another. This design was equipped with one port for culture sampling and two ports for gas inlet and outlet. Before the start of the experiment, the bags were autoclaved.
The experimental device
The experimental device (Fig. 1) consisted of a water bath to smooth out temperature fluctuations; stabilizing belts to fix the bags, temperature and light sensors connected to the QMbox modular measuring system (RG Tekhnoloji, Russia), and computer to record data. Temperature sensors were submerged in the water bath.
Cultivation conditions
In the Experiment #1, the grown cells were centrifuged (900g, 2 min) and suspended in AA/8-N medium at Chl concentration of 23.3 mg L−1. The volume of the culture in each PhBR was 1 L. The gas phase (3 L) was replaced with Ar supplemented with 5% CO2, and 3.3% of N2 was added to the PhBR 1. The cultivation was carried out in Central Russia (Moscow region, 54° 50′ N and 37° 37′ E) from July 20–25, 2016 under natural light and temperature (on the roof of a four-story building).
In the Experiment #2, the culture was not concentrated, but previously adapted to high-light intensities by gradual increase from 30 to 700 μmol photon m−2 s−1 within 4 days. The outdoor experiments began under reduced light intensity using light filters. The light filter consisted of several layers of netting, which reduced light intensity 2–7 times (the coefficient was determined in laboratory tests). Later, the number of layers varied, providing different light intensities in PhBR 3 and PhBR 4. The volume of culture in the PhBR was 1 L with initial Chl content ~ 4.3 mg L−1, the volume of the gas phase being 3 L. Air supplemented with 0.5–1.0% CO2 was successively sparged through PhBR 3 and 4 at 20–100 mL min−1. The cultivation was performed outdoors in the same place, August 18–23, 2016 (Fig. 2).
Gas analysis
In the Experiment #1, gas phase was analyzed twice a day: at 8:00 and 20:00. H2 yield and production rate were calculated based on its concentration and the volume of the gas phase of PhBR. Calculations referred to the morning (5:00–8:00), daytime (8:00–20:00), daylight (the whole light period 5:00–20:00) or the whole day (20:00–20:00). Since no H2 measurements were taken at 5:00, we assumed that at night, there was neither H2 production nor consumption, therefore, the H2 concentration obtained at 20:00 the previous day was assumed to be equal to the H2 concentration at 5:00 the next day.
In the Experiment #2, the rate of H2 evolution was calculated based on its concentration and the air flow rate, so the H2 accumulation was further calculated. In this case, we assumed that H2 concentration at night was zero due to the fast removal of previously formed H2 with air flow. The current rates of H2 evolution or averaged rates were calculated during morning, daytime, daylight or the whole day (as indicated in the captions to the figures and tables).
The concentration of H2 in the gas phase was measured at 22 °C by gas chromatography (LHM80, Moscow, Russia), equipped with a thermoconductivity detector and a 1 m × 3 mm with molecular sieve column (running temperature, 40 °C). Argon was used as the gas carrier [23]. Gas samples (0.5 mL) were withdrawn from PhBR using locked syringes and transferred to chromatograph. Hydrogen concentration was calculated on the basis of calibration using ECOCHROM program.
Analytical methods
Chl a concentration was measured spectrophotometrically at 664 nm (U–VIS 1240 Mini, Shimadzu, Japan) after extraction with 85% methanol. The light intensity (photosynthetically active radiation, 400–700 nm) was determined with a quantum meter (Quantum Meter, model: QMSW-SS, USA). To check pH value, 5 mL of culture was taken through the culture port using a sterile syringe. If necessary, pH was adjusted to 8.2 with 0.5 M NaHCO3. In both experiments, the culture was mixed manually by gently shaking the bag twice a day during sampling. The axenity of cyanobacteria culture was checked by light microscopy (LOMO MIKMED-2, Russia).
Statistical data processing
Statistical data processing was performed in the SigmaPlot 11.0 program. The average values of the results ± 95% confidence interval are presented.
Results and discussion
Outlines of weather conditions during the two experiments
Continuous monitoring of light intensity and temperature during the two experiments allowed us to characterize weather conditions. During Experiment #1 (Fig. 3a), the average daily temperature was 21.7 ± 0.4, maximal 31.4, and minimal 16.1 °C. The average daily illumination was 290 ± 40, maximal 1520, and minimal < 10 μmol photon m−2 s−1. During Experiment #2 (Fig. 3b), the average daily temperature was 20.6 ± 0.4, maximal 30.5, and minimal 14.9 °C. The average daily illumination was 340 ± 42, maximal 1650, and minimal < 10 μmol photon m−2 s−1. Hence, in Experiment #1, the illumination was lower, and in Experiment #2, the temperature was lower.
The record of the light intensity and the temperature in Experiment #1 (a, July 20–25, 2016) and in Experiment #2 (b, August 18–23, 2016). The start of Experiment #1 was at 14:00, the first measurement was at 20:00 (0 point). The start of Experiment #2 was at 17:00 (0 point), the first measurement was at 13:00 the next day (point 20 h)
The hydrogen production in Experiment #1
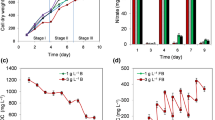
As indicated above, both the PhBRs were initially filled with Ar + CO2, but 3.3% of N2 was added to the gas phase of PhBR 1. The patterns of H2 accumulation were similar in both PhBRs (Fig. 4a). The maximum rates of H2 production were at midday, but they varied over the observation period (Fig. 4b). The decrease in the maximum rate at 40th and 64th h correlated with the low daytime temperature and illumination (Fig. 4a).
The average rate of H2 production in the daytime in PhBRs 1 and 2 increased with the average light intensity (Fig. 5a). Similar dependence was shown in other outdoor experiments [16]. In more detail, the influence of light intensity was studied in Experiment #2.
A decrease in the average rate of H2 evolution in the morning was observed with a decrease in temperature in the morning hours (Fig. 5b) or during the preceding night (not shown). Earlier, a low daily temperature was supposed to be the main limiting factor in obtaining H2 outdoors [16]. A decrease in H2 production activity with decreasing temperature is a matter of common knowledge [24, 25]. However, the effect should not be as dramatic as shown in Fig. 5b: about 10 times decrease was seen in H2 production rate with temperature decrease by only 3 °C. We would like to attribute it to the mutual effect of low temperature and high-light intensity. Earlier in short-term laboratory experiments, we showed that at 16 °C, the photosynthetic activity of this strain was inhibited at light intensity above 300 μmol photon m−2 s−1, while at higher temperatures no inhibition was observed even at 2800 μmol photon m−2 s−1 [25]. Although the average light intensity in the morning was only about 100 μmol photon m−2 s−1 and the average morning temperature was 15.1–16.6 °C, the duration of the impact was much longer. Therefore, the photoinhibition may be one of the explanations for this dependence.
Torzillo and Vonshak [26] demonstrated that photoinhibition of photosynthetic activity was much higher at temperatures below and above the optimum, in the open ponds or closed systems, correspondingly. Outdoor experiments with Synechocystis PCC 6803 in 50 L tubular PhBR showed that photoinhibition strongly affected maximum quantum yield of PSII at low biomass concentration [17].
The presence of N2 in the Ar gas phase might be helpful for prolonged H2 production during 20 days as was shown earlier [27]. However, in our rather short experiments, it significantly affected neither the current H2 production rates, nor its accumulation (Fig. 4a, b).
It should be noted that the final N2 concentration in PhBR 2 (without N2 addition) did not exceed 0.3%, which proves that these bags have negligible permeability to at least N2. We cannot judge the permeability to H2 based on our data, but the previous investigation shows that it was very low [20]. The final oxygen concentration was ~ 10% (Fig. 4c), i.e., lower than that in the air and due evidently to the photosynthetic activity of cyanobacteria. The maximum increase in O2 concentration was observed in the first 2 days of the experiment, later the O2 concentration did not increase (Fig. 4c). This could be due to a decrease in photosynthetic activity, therefore 40 mL of 0.5 M NaHCO3 was added at 113th h (not shown). However, O2 evolution did not resume, and H2 evolution ceased. Perhaps this was due to inadequate mixing, because control pH measurement (7.2) turned out to be incorrect—actual pH was 10.2, which was the reason for experiment termination. Mixing technique in PhBR of such type should be thought out. It is well-known that appropriate stirring strongly affected the biomass output of cyanobacteria [28]. However, for a large-scale cultivation in the future, mixing seems to be improbable to execute.
The hydrogen production in Experiment #2
Since in Experiment #1, both PhBRs showed similar results, the influence of light intensity (four different values) was studied in parallel in two PhBRs (two values for each PhBR). The light intensity was changed using filters as indicated in Materials and Methods.
Both the maximum and average (per daylight) H2 production rates were the lowest in the unshadowed culture at 1400 µmol photon m−2 s−1 (Table 1), which suggested the possibility of photoinhibition. It is well-known that the effect of light intensity may be different in various strains of cyanobacteria [10]. For example, low-light intensity was recommended for laboratory H2 production by some filamentous cyanobacteria [29, 30]. Besides, this effect may also depend on other factors: the cell concentration, duration of illumination and range of the light intensities. Data of Experiment #2 (Table 1) differ from those of Experiment #1 (Fig. 5a), where no light inhibition was observed. We can ascribe it to lower-light intensities, higher Chl concentration (~ 20 mg Chl L−1) as well as lower O2 concentration (due to Ar gas phase) in Experiment #1. Our data (Fig. 5a) referred to prolonged H2 production. When cell suspension of the same culture in short-term (2 min) experiments was tested, we observed no inhibition even at 2800 µmol photon m−2 s−1 [25]. In short-term experiments, the Hup− mutant of A. variabilis PK84 demonstrated only a slight sensitivity to O2 concentration [31]. In our Experiment #2 at various light intensities, there were no essential differences in O2 concentrations measured against the background of air flow (not shown). However, since the air flow was delivered above the surface, the intimate conditions in culture may be different and pO2 may be elevated. Generally speaking, the light delivery to the culture was inhomogeneous due to absence of continuous mixing [28]. In a well-mixed outdoor culture A. variabilis PK84, the H2 rate did not decrease but conversion efficiency of light/H2 energy decreased at high daily irradiance [16].
Summary of two experiments
Experiments #1 and #2 were carried out with different initial Chl concentrations (Table 2). However, an increase in Chl concentration in the course of the experiment was not observed, there was even a slight decrease. This could be explained by a decrease in the specific content of Chl due to high natural light intensity, which was noted earlier [32]. Indeed, OD (another growth indicator), in contrast to Chl concentration, increased by approximately 50% during Experiment #2 (PhBRs 3, 4). This can be due to accumulation of saccharides. On the other hand, an increase in OD with a decrease in Chl content may also indicate bacteriological contamination of the culture. Such a change in parameters in Experiment #2 (PhBR 4) was actually accompanied by contamination, which was confirmed by microscopy after 110 h (not shown). At the same time, pO2 also decreased, and the H2 production ceased, which were subsequently not restored (therefore, the experiment has been stopped).
The average rate of H2 production did not exceed 7.2–7.4 mL L−1 day−1 during 5 days, whereas the maximal volumetric H2 production rate amounted to 18.5–20.6 mL L−1 day−1 (Table 2), but it took place only for a few hours (2–4 h). The maximal rate per 1 mg Chl amounted to 3.8 mL day−1 in Experiment #2 with lower Chl content. Hydrogen accumulation reached 33.2 mL L−1. Compared to the best outdoor experiments [14, 16], the rather short duration and quite low values of H2 production in our study can be due to several reasons: the simple PhBR design, poor mixing and culture heterogeneity, and uncontrolled pH. Natural conditions were not optimal as well. Contamination was still a problem even if cyanobacteria were cultivated with antibiotics. As a disadvantage, we should mention poor reliability of plastic bags when using without mechanical protection. Actually, some bags were rejected after the start of experiments due to damage.
We would like to emphasize that during almost 2 decades, there were only a few attempts of outdoor H2 production using filamentous or unicellular cyanobacteria [14, 16, 17]. Despite the successful laboratory experiments and theoretical considerations, actual outdoor H2 production is still a challenge. It seems that practical devices need to be more complicated and cost consuming to provide reliable hydrogen. Besides, weather conditions (geographic region) should be chosen carefully. In general, our very first attempt in outdoor cultivation proves that photoautotrophic H2 production by cyanobacteria in Moscow region is feasible, however, there is still great room for improvement.
Conclusions
Our experiments prove for the first time that H2 production by cyanobacteria in cheap plastic bags is feasible outdoors in Moscow region in the middle-late summer. However, the rates and yields were lower than in best experiments described earlier. We suggest that rather low H2 production in our outdoor experiments can be attributed to several reasons: suboptimal weather conditions (low night temperatures, high daytime illumination), unsuitable culture conditions (heterogeneity due to inappropriate mixing) and contamination.
References
Sakurai, H., Masukawa, H., Kitashima, M., Inoue, K.: A feasibility study of large-scale photobiological hydrogen production utilizing mariculture-raised cyanobacteria. Adv. Exp. Med. Biol. 675, 291–303 (2010)
Sakurai, H., Masukawa, H., Kitashima, M., Inoue, K.: Photobiological hydrogen production: bioenergetics and challenges for its practical application. J. Photochem. Photobiol. C Photochem. Rev. 17, 1–25 (2013)
Tsygankov, A.A., Khusnutdinova, A.N.: Hydrogen in metabolism of purple bacteria and prospects of practical application. Microbiology 84(1), 1–22 (2015)
Hallenbeck, P.C., Liu, Y.: Recent advances in hydrogen production by photosynthetic bacteria. Int. J. Hydrogen Energy 41(7), 4446–4454 (2016)
Sakurai, H., Tsygankov, A.A.: Chapter 16. Photobiological biohydrogen production. In: Basile, A., Dalena, F. (eds.) Second and Third Generation of Feedstocks. Elsevier, New York (2019)
Deprá, M.C., Mérida, L.G.R., de Menezes, C.R., Zepka, L.O., Jacob-Lopes, E.: A new hybrid photobioreactor design for microalgae culture. Chem. Eng. Res. Des. 144, 1–10 (2019)
Tredici, M.: Mass production of microalgae: photobioreactors. In: Richmond, A. (ed.) Handbook of Microalgal Culture: Biotechnology and Applied Phycology, pp. 178–214. Blackwell Publishing, Oxford (2004)
Ugwu, C.U., Aoyagi, H., Uchiyama, H.: Photobioreactors for mass cultivation of algae. Bioresour. Technol. 99, 4021–4028 (2008)
Sakurai, H., Masukawa, H., Kitashima, M., Inoue, K.: How Close we are to achieving commercially viable large-scale photobiological hydrogen production by cyanobacteria: a review of the biological aspects. Life 5(1), 997–1018 (2015)
Dutta, D., De, D., Chaudhuri, S., Bhattacharya, S.: Hydrogen production by cyanobacteria. Microb. Cell Fact. 4(1), 1–11 (2005)
Happe, T., Schutz, K., Bohme, H.: Transcriptional and mutational analysis of the uptake hydrogenase of the filamentous cyanobacterium Anabaena variabilis ATCC 29413. J. Bacteriol. 182(6), 1624–1631 (2000)
Yoshino, F., Ikeda, H., Masukawa, H., Sakurai, H.: High photobiological hydrogen production activity of a Nostoc sp. PCC 7422 uptake hydrogenase-deficient mutant with high nitrogenase activity. Mar. Biotechnol. 9(1), 101–112 (2007)
Fernández-Sevilla, J.M., Acién-Fernández, F.G., Molina-Grima, E.: Photobioreactors design for hydrogen production. In: Zannoni, D., De Philippis, R. (eds.) Microbial Bioenergy: Hydrogen Production, pp. 291–320. Springer Science + Business Media, Dordrecht (2014)
Lindblad, P., Christensson, K., Lindberg, P., Fedorov, A., Pinto, F., Tsygantov, A.A.: Photoproduction of H2 by wildtype Anabaena PCC 7120 and a hydrogen uptake deficient mutant: from laboratory experiments to outdoor culture. Int. J. Hydrogen Energy 27(11–12), 1271–1281 (2002)
Borodin, V.B., Tsygankov, A.A., Rao, K.K., Hall, D.O.: Hydrogen production by Anabaena variabilis PK84 under simulated outdoor conditions. Biotechnol. Bioeng. 69(5), 478–485 (2000)
Tsygankov, A.A., Fedorov, A.S., Kosourov, S.N., Rao, K.K.: Hydrogen production by cyanobacteria in an automated outdoor photobioreactor under aerobic conditions. Biotechnol. Bioeng. 80(7), 777–783 (2002)
Touloupakis, E., Benavides, A.M.S., Cicchi, B., Torzillo, G.: Growth and hydrogen production of outdoor cultures of Synechocystis PCC 6803. Algal Res. 18, 78–85 (2016)
Amos, W.A.: Updated cost analysis of photobiological hydrogen production from Chlamydomonas reinhardtii green algae—milestone completion report. NREL/MP-560-35593 (2004). http://www.nrel.gov.docs.fy04osti/35593.pdf. Accessed 27 Jan 2011
Sakurai, H., Masukawa, H.: Promoting R & D in photobiological hydrogen production utilizing mariculture-raised cyanobacteria. Mar. Biotechnol. 9(2), 128–145 (2007)
Kitashima, M., Masukawa, H., Sakurai, H., Inoue, K.: Flexible plastic bioreactors for photobiological hydrogen production by hydrogenase-deficient cyanobacteria. Biosci. Biotechnol. Biochem. 76(4), 831–833 (2012)
Masukawa, H., Mochimaru, M., Sakurai, H.: Hydrogenases and photobiological hydrogen production utilizing nitrogenase system in cyanobacteria. Int. J. Hydrogen Energy 27(11–12), 1471–1474 (2002)
Allen, M.B., Arnon, D.I.: Studies on nitrogen-fixing blue-green algae. I. Growth and nitrogen fixation by Anabaena cylindrica Lemm. Plant. Physiol. 30(4), 366–372 (1955)
Laurinavichene, T., Tekucheva, D., Laurinavichius, K., Ghirardi, M., Seibert, M., Tsygankov, A.: Towards the integration of dark and photo fermentative waste treatment. 1. Hydrogen photoproduction by purple bacterium Rhodobacter capsulatus using potential products of starch fermentation. Int. J. Hydrogen Energy 33(23), 7020–7026 (2008)
Jensen, B.B., Cox, R.P.: Direct measurements of steady-state kinetics of cyanobacterial N2 uptake by membrane-leak mass spectrometry and comparisons between nitrogen fixation and acetylene reduction. Appl. Environ. Microbiol. 45(4), 1331–1337 (1983)
Romanova, A.I., Laurinavichene, T.V., Tsygankov, A.A.: Features of Anabaena PCC 7120ΔHup mutants with amino acid substitutes in nitrogenase. Plant Physiol. (2020) (in press)
Torzillo, G., Vonshak, A.: Effect of light and temperature on the photosynthetic activity of the cyanobacterium Spirulina platensis. Biomass Bioenergy 6(5), 399–403 (1994)
Masukawa, H., Sakurai, H., Hausinger, R.P., Inoue, K.: Sustained photobiological hydrogen production in the presence of N2 by nitrogenase mutants of the heterocyst-forming cyanobacterium Anabaena. Int. J. Hydrogen Energy 39(34), 19444–19451 (2014)
Richmond, A.: Mass culture of cyanobacteria. In: Mann, N.H., Carr, N.G. (eds.) Photosynthetic Prokaryotes, pp. 181–208. Plenum Press, New York (1992)
Vargas, S.R., dos Santos, P.V., Zaiat, M., Calijuri, M.C.: Optimization of biomass and hydrogen production by Anabaena sp. (UTEX1448) in nitrogen-deprived cultures. Biomass Bioenergy 111, 70–76 (2018)
Yodsang, P., Raksajit, W., Aro, E.-M., Mäenpää, P., Incharoensakdi, A.: Factors affecting photobiological hydrogen production in five filamentous cyanobacteria from Thailand. Photosynthetica 56(1), 334–341 (2018)
Tsygankov, A., Serebryakova, L., Rao, K., Hall, D.: Acetylene reduction and hydrogen photoproduction by wild type and mutant strains of Anabaena at different CO2 and O2 concentrations. FEMS Microbiol. Lett. 167(1), 13–17 (1998)
Marques, A.E., Barbosa, A.T., Jotta, J., Coelho, M.C., Tamagnini, P., Gouveia, L.: Biohydrogen production by Anabaena sp. PCC 7120 wild-type and mutants under different conditions: light, nickel, propane, carbon dioxide and nitrogen. Biomass Bioenergy 35(10), 4426–4434 (2011)
Acknowledgements
Authors thank Prof. Inoue, K. for presenting to us the used strain of cyanobacterium. Experimental data acquisition was supported by the Russian Foundation of Basic Research No 15-54-50032, whereas data analysis and the paper preparation were supported by Russian Ministry of Science and Education (AAAA-A17-117030110141-2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shastik, E., Romanova, A., Laurinavichene, T. et al. Plastic bags as simple photobioreactors for cyanobacterial hydrogen production outdoors in Moscow region. Int J Energy Environ Eng 11, 1–8 (2020). https://doi.org/10.1007/s40095-019-00325-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40095-019-00325-0