Abstract
A controlled “green synthesis” approach to synthesize silver nanoparticles by Allium cepa and Musa acuminata plant extract has been reported. The effect of different process parameters, such as pH, temperature and time, on synthesis of Ag nanoparticles from plant extracts has been highlighted. The work reports an easy approach to control the kinetics of interaction of metal ions with reducing agents, stabilized by ammonia to achieve sub-10 nm particles with narrow size distribution. The nanoparticles have been characterized by UV–Visible spectra and TEM analysis. Excellent antimicrobial activity at extremely low concentration of the nanoparticles was observed against Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis and Fusarium oxysporum which may allow their exploitation as a new generation nanoproduct in biomedical and agricultural applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanomaterials are going radical due to their exceptional performance directing their application in everyday products such as water filters, cosmetics, packaging materials and coating over surfaces. Exposure to nanomaterials used in these products may lead to internalization of nanomaterials in our body. Nanomaterial synthesis is one of the promising fields which keeps on growing radically and many synthesis routes have been devised to achieve nanoscale materials. But, unfortunately most of these methods make use of toxic organic solvents which make the nanoparticles applicability almost impossible for human use. Although, conventional methods for synthesis of nanomaterials can make attainment of desired properties possible, but their toxicity may be compromised for it. Severe toxicity has been reported for various nanoparticles. Especially in biomedical, nanomaterials were designed for various applications such as fluorescent nanomaterials for disease diagnosis [1, 2], tissue engineering [3], bioimaging [4], biosensors [5] and implants [6]. Exposure to toxic nanomaterials will have critical implications, due to which green methods for synthesis of nanoparticles should be devised for sustainable and green development. The accomplishment of future developments with the aid of nanotechnology requires a sustainable approach that can minimize the involvement of hazardous substances and can carefully integrate the future technology with clean environment. The efforts can be approached by identifying and utilizing “green synthesis methods” that correlate in providing higher efficacy of chemical processes with the help of natural, nontoxic and environmental benign solvents [7].
Silver nanoparticles (Ag NPs) are classified metal nanoparticles with distinctive properties such as strong biological activity, good catalytic ability and excellent electrical and optical properties [8]. The unique properties of Ag NPs have promoted their incorporation into products that range from photovoltaics [9, 10], optical sensors [11] and conductive inks [12] to medicine [12, 13]. Silver, both at micro and nanoscale, has been known for its excellent antimicrobial action against a broad spectrum of microorganisms [14, 15]. Nanosized silver particles offer a more pronounced antimicrobial action owing to their large surface-to-volume ratio, providing greater interaction with microbial cells and surprisingly decreased toxicity to human beings [16–19]. This potential of Ag NPs can be utilized in management and controlling of animal and plant pathogens in a relatively safer way compared to synthetic antibiotics and fungicides.
Stable silver nanoparticles can be synthesized by chemical methods such as chemical reduction, electrochemical techniques and photochemical reduction [8, 20, 21]. Recently, the green synthesis approach to fabricate silver nanoparticles has gained interest, owing to its environment friendly aspect. These methods include polyoxometalates method [22], polysaccharide method [23], Tollens method [24], irradiation method [25] and biological approaches [26–28]. Among these, the plant-mediated synthesis provides more efficient technique compared to other available methods, as it involves the use of environmental friendly solvents, is cost effective, can easily be scaled up and does not involve complex thermodynamic conditions during the synthesis. The extracts from different plant species, such as Azadirachta indica [29], Aloe vera [30], Medicago sativa [31], and Desmodium triflorum [32], are being used for the synthesis of silver nanoparticles with varied size, shapes and morphologies. The characteristic features of these nanoparticles are strongly influenced by the experimental conditions as well as the kinetics of interaction of metal ions with reducing agents. In chemical method for synthesis of silver nanoparticles, the effect of ammonia has been studied and proven to act as a stabilizer for silver nanoparticles. Ammonia plays a key role in synthesis of silver nanoparticles which has been used as a stabilizer in the present study [33].
The present study highlights the controlled synthesis of silver nanoparticles from Allium cepa and Musa acuminata extracts in ammonia stabilized conditions. The role of different process parameters, such as pH, temperature and time, has been emphasized with control in the kinetics of interaction of metal ions with reducing agents aided by ammonia. This control mechanism can provide a solution to polydispersity and higher size range on silver nanoparticles obtained from plant extracts. Further, the antimicrobial activity of silver nanoparticles is studied against Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis and Fusarium oxysporum. Antifungal activity of silver nanoparticles against F. oxysporum infecting growing seedling of Vigna radiata and Ceci neri has been investigated ex vivo.
Materials and methods
Materials
Silver nitrate (AgNO3), purchased from S.D. fine chemicals ltd., India, was used as a source of silver. Ammonia solution (30 %, NH3·H2O) was purchased from Loba chemicals, India. Agar, Luria–Bertani (LB) medium for bacterial cultures and Potato dextrose broth for fungal cultures were obtained from HiMedia chemicals, India. All the chemicals were of analytical grade and were used without further purification. The solutions were prepared using Millipore® water. The plant extracts were prepared from bulb of A. cepa and leaves of M. acuminata. The pure cultures of E. coli (MTCC No. 729), B. Subtilis (MTCC No. 736), P. aeruginosa (MTCC No. 4637) and F. oxysporum (MTCC No. 3656) for antimicrobial studies were purchased from Microbial Type Culture Collection and Gene Bank (MTCC) facility, IMTECH, Chandigarh, India.
Synthesis of silver nanoparticles
The plant extract was prepared using A. cepa bulb and Musa acuminate leaves. In a typical reaction set, 30 gm of respective plant components was finely chopped and boiled for 2–3 min in 100 ml of distilled water. The extract was filtered by Whatman filter paper and was used for the synthesis of silver nanoparticles. A final volume of 20 ml was prepared by adding 5 mM of silver nitrate (AgNO3), 3 ml of extract and 1 ml of 30 % Ammonia in Millipore® water. The procedure followed was a slight variation to the method reported by Chandran et al. [30] for synthesis of gold nanostructures using Aleo vera plant extract. The reaction was performed in dark conditions. The reaction was optimized and stabilized by varying pH, temperature and time conditions during the experiment. The pH was varied from 4 to 12 and temperature was varied from 5 to 50 °C. The absorbance spectra were recorded after appropriate time intervals to optimize the optimum time required for the synthesis of Ag NPs. The formation of silver nanoparticles was indicated by the formation of yellowish-brown color of silver nanocolloid.
Antimicrobial activity of silver nanoparticles
For antibacterial and antifungal studies, silver nanoparticles prepared using A. cepa extract at pH 12 and 40 °C for 48 h were chosen. The antimicrobial activity of Ag NPs was studied by Minimum Inhibitory Concentration (MIC) test. The quantitative analysis was performed by growing E. coli, B. subtilis, P. aeruginosa (108 CFU) in LB broth medium and F. oxysporum (108 CFU) in Potato dextrose broth medium containing different concentrations of Ag NPs (10, 20, 30, 40, 50, 60 l/ml corresponding to 5.35, 10.7, 16.05, 21.4, 26.75, 32.10 g/ml of Ag NPs, respectively). The E. coli, B. subtilis, P. aeruginosa and F. oxysporum cells treated with different concentrations of the Ag NPs were incubated at 37 °C for 18 h and 28 °C for 48 h, respectively. The cells grown in absence of the Ag NPs were taken as the microbial control and the cells grown only in extract were taken as the extract control. To investigate the antimicrobial activity, optical density of the samples was recorded at 600 nm [34].
Antifungal activity of Ag NPs in agar medium (DAT test)
The direct inhibition of infection by plant pathogen, F. oxysporum on V. radiata (moong dal) and C. neri (black chickpea) in the presence of silver nanoparticles was conducted using dual agar test (DAT) test [35]. In typical procedure, the Petri dish test unit containing 15 ml of agar culture media (10 ml of 0.5 % agar and 5 ml of 0.25 % agar) along with a different concentration of nanoparticles (10, 20, 30, 40 l/ml corresponding to 5.35, 10.7, 16.05, 21.4 g/ml of Ag NPs, respectively) was prepared. Each petridish was inoculated with 50 l/ml of F. oxysporum (108 CFU). Further, sterilized seeds were subsequently placed onto the agar and kept for appropriate time in incubator at a temperature of 25 °C. The test seeds grown in the absence of the Ag NPs were taken as a microbial control (control 1) and the test seeds grown in absence of F. oxysporum were taken as seed control (control 2). The growth responses of F. oxysporum and growing seedlings were analyzed.
Characterization methods
UV–Visible spectra of the silver nanoparticles were observed using a Shimadzu UV-3600 UV–VIS–NIR spectrophotometer in the wavelength range of 200–800 nm. A TECNAI TEM (Fei, Electron Optics) and Hitachi (H-7500) transmission electron microscope (TEM) operating at 200 kV were used to study the morphology and size of the silver nanoparticles. Optical density (OD) measurements were performed at 600 nm using an Elico SL 159 UV–Visible spectrophotometer.
Results and discussion
The aqueous solution of silver nitrate acts as a source of silver ions for the synthesis of silver nanoparticles. The silver ions were reduced to silver atoms by extracts of A. cepa and Musa acuminate which nucleate to form nano-crystallites for growth. The formation of silver nanoparticles was indicated by change in color of the medium from colorless to yellow; yellow color turns to dark brown with an increase in the concentration of silver nanoparticles (Fig. 1) [36, 37]. The confirmation of synthesis of Ag NPs in colloidal solution was monitored by the presence of surface plasmon resonance (SPR) band in the absorbance spectra recorded by UV–Vis spectrophotometer (shown later). The primary characterization of silver nanoparticles by absorbance spectral studies has proven to be a successful technique for the analysis of metal nanoparticles [38]. A defined sharp band, with absorbance maxima at 401 and 420 nm for silver nanoparticles synthesized by A. cepa and M. acuminata, respectively, confirmed the formation of nanoparticles in colloidal state [39].
The formation and stabilization of silver nanoparticles by plant extracts are attributed to the ionic or electrostatic interactions between the metal complexes and the organic functional groups including flavonoids, terpenoids, proteins, reducing sugars and alkaloids, present on the biomass surface [40]. The conversion mechanism of enol to ketone group in flavonoids liberates reactive hydrogen which plays a significant role in the reduction of metal ions (Ag+ to Ag0) [35]. A variety of phytochemical compounds, such as quercetin, isorhamnetin and phenols, present in extract of A. cepa, are expected to reduce and cap the metal ions formed in their presence, thereby inducing shape control during metal ion reduction [32, 41]. The major flavonoids and terpenoids identified in M. acuminata, such as Luteolin and Apigenin and corosolic acid, are active reducing species during the synthesis of silver nanoparticles [42].
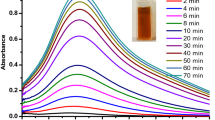
The effect of different parameters, including pH, temperature and the reaction time on the formation of silver nanoparticles from plant extract, was analyzed as an important factor in the process of reduction. The optimum time for the synthesis of stable colloid of Ag NPs was analyzed by recording the absorbance spectra of samples withdrawn from the reaction mixture at regular time intervals (Fig. 2). SPR band formation was indicative after 6 h of start of reaction with a continuous rise till 72 h. A sharp band was successfully achieved at 48 h indicating adequate time for controlled synthesis of Ag NPs.
Significant effect of temperature was observed on the synthesis of silver nanoparticles by A. cepa and M. acuminata. A marked variation in plasmon band intensity was observed at different temperature conditions, 5–50 °C (Fig. 3). A sharp increase in plasmon maxima at 401 nm was recorded with increase in temperature to 40 °C, which showed a sharp decrease as the temperature was increased to 50 °C. The effect of temperature was also indicated by a deep brown color at 40 °C compared to yellow and whitish yellow at other temperature conditions (Fig. 1). This may be due to favorable reduction and interaction conditions provided by flavonoids and terpenoids in the extract at this particular temperature condition.
Another important parameter during the Ag NPs biosynthesis was pH. The pH of the reaction mixture can significantly alter the reducing conditions of the environment playing a major role in the synthetic procedure. The synthesis of Ag NPs was observed only in high basic conditions at pH 12 and pH 11–12 by A. cepa and M. acuminata (Fig. 4). This was also observed by the absence of yellowish brown color at lower pH condition that turn out to be completely unfavorable for biosynthesis (not shown).
The size and morphology of Ag NPs synthesized from A. cepa and M. acuminata at 40 °C and pH 12 were characterized by TEM. The shape of Ag NPs synthesized by A. cepa extract was spherical with the size ranging in sub-10 nm (Fig. 5a). The particles exhibited monodispersity and were observed to be free as well as attached with the organic components of A. cepa extract (Fig. 5b). The Ag NPs synthesized from M. acuminata were also of spherical morphology with slightly higher nanometer range than the above but were monodispersed (Fig. 5c).
Around 150 particles were analyzed for the calculation of the particle size. The maximum particles were of 1–4 nm with a sharp size distribution ranging between 1 and 10 nm for Ag NPs synthesized by A. cepa extract (Fig. 5d). The particle size distribution for Ag NPs synthesized from M. acuminata was ranging from 15 to 25 nm. The significant attribute of sharp size distribution is the “ammonia solution” added during the synthesis of Ag NPs. Ammonia acts as an entrapment system for free silver ions in the system after the nucleation step, thereby preventing particle growth and the generation of new nuclei. Due to ammonia, the reaction is freezed in the initial stage as the free silver ion is converted to soluble diamine silver (I) complexes, thus preventing the formation of new nuclei and the growth of already formed nanoparticles [33]. This can eventually result in virtually monodisperse silver nanoparticles (Fig. 6).
As supported by the above characterization and analysis, the Ag NPs synthesized from A. cepa at 40 °C and pH 12 were considered as best optimized condition and were chosen for studying the antimicrobial activity against E. coli, B. subtilis, P. aeruginosa and F. oxysporum. The antimicrobial activity of Ag NPs was readily analyzed by conventional minimum inhibitory concentration (MIC) broth assay. The microbial strains inoculated in appropriate growth medium and treated with different concentrations of Ag NPs were incubated for adequate time and optical density was recorded at 600 nm giving direct measure of microbial cell density. For bacterial strains, namely, E. coli, B. subtilis and P. aeruginosa, the growth retarded at Ag NPs concentration of 10.7 μg/ml with complete inhibition at 16.05 μg/ml and further (Fig. 7a). This indicated a strong antibacterial activity of Ag NPs synthesized by A. cepa with MIC at 16.05 μg/ml. The MIC of Ag NPs synthesized by A. cepa against F. oxysporum was 32.1 μg/ml (Fig. 7b). The microbial cells treated with only A. cepa extract showed no growth inhibition, clearly indicating the role of Ag NPs in the inhibition microbial cells.
The inhibitory effect of Ag NPs on plant pathogen, F. oxysporum, during the plant growth was evaluated by DAT. The growth of F. oxysporum was analyzed by the presence of white cottony appearance on the growing plant seedling in the agar unit. As evident from Figs. 8 and 9, attack of F. oxysporum on growth of V. radiata and C. neri seedling was completely inhibited in the presence of 10.7 μg/ml concentration of Ag NPs. The growth of plant seeds in the absence of Ag NPs and F. oxysporum was set as control reactions for correct interpretation of the result.
Conclusions
A novel “green” approach to synthesis silver nanoparticles from A. cepa and M. acuminata has been optimized. The effect of pH, temperature and time on synthesis of silver nanoparticles is clearly demonstrated from the results. The optimum conditions for synthesis of stable silver nanocolloid are represented to be 40 °C at pH 12 for 48 h. The kinetics of the reaction can be controlled by incorporation of appropriate amount of ammonia solution to achieve sub-10 nm particles of silver with narrow size distribution. Strong antibacterial and antifungal activity of silver nanoparticles, synthesized by A. cepa extract, with minimum inhibitory concentration (MIC) of 16.05 and 32.10 μg/ml has been observed. To the best of author’s knowledge, the present study first time reports the inhibitory effect of Ag NPs at low concentration of 10.7 μg/ml on plant pathogen, F. oxysporum ex vivo, during the plant seedling growth evaluated by DAT. The report, therefore, directs the study to be explored for generation of nanoproducts by environmental friendly methods for application in biomedical and agricultural fields.
References
Zhang, Q., Iwakuma, N., Sharma, P., Moudgil, B.M., Wu, C., Neill, J., Jiang, H., Grobmyer, S.R.: Gold nanoparticles as a contrast agent for in vivo tumor imaging with photoacoustic tomography. Nanotech. 29, 395102 (2009)
Panwar, A., Yadav, K.L.: A novel one-pot synthesis of hierarchical europium doped ZnO nanoflowers. Mat. Lett. 142, 30–34 (2015)
Zhang, L., Webster, T.J.: Nanotechnology and nanomaterials: promises for improved tissue regeneration. 4, 66–80 (2009)
Takuya, T., Tensuo, T.: Fluorescent probes for bioimaging applications. Curr. Opin. Chem. Biol. 12, 515–521 (2008)
Zhang, G.-H., Zhang, L., Huang, M.J., Luo, Z.H.H., Tay, G.K.I., Lim, E.J.A., Kang, T.G., Chen, Y.: Silicon nanowire biosensor for highly sensitive and rapid detection of Dengue virus. Sens Actuators A 146, 138–144 (2010)
Jayakumar, R., Deepthy, D., Manzoor, K., Nair, S.V., Tamura, H.: Biomedical applications of chitin and chitosan based nanomaterials—a short review. Carbohydr. Polym. 82, 227–232 (2010)
Anstas, P.T., Warner, J.C.: Green Chemistry: Theory and Practice. Oxford University Press Inc, New York (1998)
Frattini, A., Pellegri, N., Nicastro, D., de Sanctis, O.: Effect of amine groups in the synthesis of Ag nanoparticles using aminosilanes. Mater. Chem. Phys. 94, 148–152 (2005)
Yoon, W.J., Jung, K.Y., Liu, J., Duraisamy, T., Revur, R., Teixeira, F.L., Sengupta, S., Berger, P.R.: Plasmon-enhanced optical absorption and photocurrent in organic bulk heterojunction photovoltaic devices using self-assembled layer of silver nanoparticles. Sol. Energy Mater. Sol. Cells 94, 128–132 (2010)
Naidu, B.V.K., Park, J.S., Kim, S.C., Park, S.M., Lee, E.J., Yoon, K.J., et al.: Novel hybrid polymer photovoltaics made by generating silver nanoparticles in polymer:fullerene bulk-heterojunction structures. Sol Energy Mat Sol C 92, 397–401 (2008)
McFarland, A.D., Van Duyne, R.P.: Single silver nanoparticles as real-Time optical sensors with zeptomole sensitivity. Nano Lett. 3, 1057–1062 (2003)
Ankireddy, K., Vunnam, S., Kellar, J., Cross, W.: Highly conductive short chain carboxylic acid encapsulated silver nanoparticle based inks for direct write technology applications. J. Mater. Chem. C 1, 572–579 (2013)
Samuel, U., Guggenbichler, J.P.: Prevention of catheter—related infections: the potential of a new nanosilver impregnated catheter. Int. J. Antimicrob. Ag. 23, 75–78 (2004)
Roe, D., Karandikar, B., Bonn-Savagel, N., Gibbins, B., Roullet, J.B.: Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J. Antimicrob. Chemother. 61, 869–876 (2008)
Morones, J.R., Elechiguerra, J.L., Camacho, A., Holt, K., Kouri, J.B., Ramirez, J.T., Yacaman, M.J.: The bactericidal effect of silver nanoparticles. Nanotechnology 16, 2346–2353 (2005)
Shrivastava, S., Bera, T., Roy, A., Singh, G., Ramachandrarao, P., Dash, D.: Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 18, 225103 (2007)
Lok, C.N., Ho, C.M., Chen, R., He, Q.Y., Yu, W.Y., Sun, H., Tam, P.K.H., Chiu, J.F., Che, C.M.: Silver nanoparticles: partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 12(4), 527–534 (2007)
Alt, V., Bechert, T., Steinrucke, P., Wagener, M., Seidel, P., Dingeldein, E., Domann, E., Schnettler, R.: An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials 25(18), 4383–4391 (2004)
Foldbjerg, R., Olesen, P., Hougaard, M., Dang, D.A., Hoffmann, H.J., Autrup, H.: PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol. Lett. 190(2), 156–162 (2009)
Khan, Z., Al-Thabaiti, S.A., Obaid, A.Y., Al-Youbi, A.O.: Preparation and characterization of silver nanoparticles by chemical reduction method. Colloids Surf. B 82, 513–517 (2011)
Chen, W., Cai, W., Zhang, L., Wang, G., Zhang, L.: Sonochemical processes and formation of gold nanoparticles within pores of mesoporous silica. J. Colloid Interface Sci. 238(2), 291–295 (2001)
Troupis, A., Hiskia, A., Papaconstantinou, E.: Synthesis of metal nanoparticles by Using polyoxometalates as photocatalysts and stabilizers. Angew. Chem. Int. Ed. 41, 1911–1914 (2002)
Raveendran, P., Fu, J., Wallen, S.L.: Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc. 125(46), 13940–13941 (2003)
Sato, Y., Wang, J.J., Batchelder, D.N., Smith, D.A.: Simple chemical method for forming silver surfaces with controlled grain sizes for surface plasmon experiments. Langmuir 19, 6857–6861 (2003)
Abid, J.P., Wark, A.W., Brevet, P.F., Girault, H.H.: Preparation of silver nanoparticles in solution from a silver salt by laser irradiation. Chem. Commun. 7, 792–793 (2002)
Collera-Zuniga, O., Jimenez, F.G., Gordillo, R.M.: Comparative study of carotenoid composition in three mexican varieties of Capsicum annuum. Food Chem. 90, 109–114 (2005)
Jagadeesh, B.H., Prabha, T.N., Srinivasan, K.: Activities of β-hexos-aminidase and a-mannosidase during development and ripening of bell capsicum (Capsicum annuum var. variata). Plant Sci. 167, 1263–1271 (2004)
Xie, J., Lee, J.Y., Wang, D.I.C., Ting, Y.P.: Silver nanoplates: from biological to biomimetic synthesis. ACS Nano. 1(5), 1429–1439 (2007)
Shankar, S.S., Rai, A., Ahmad, A., Sastry, M.: Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 275, 496–502 (2004)
Chandran, S.P., Chaudhary, M., Pasricha, R., Ahmad, A., Sastry, M.: Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 22, 577–583 (2006)
Gardea-Torresdey, J.L., Gomez, E., Peralta-Videa, J.R., Parsons, J.G., Troiani, H., Yacaman, M.J.: Alfalfa sprouts: a natural source for synthesis of silver nanoparticles. Langmuir 19, 1357–1361 (2003)
Ahmad, N., Sharma, S., Singh, V.N., Alam, M.K., Mehta, B.R., Fatma, A.: Biosynthesis of silver nanoparticles from desmodium triflorum: a novel approach towards weed utilization. Colloids Surf. B 81(1), 81–86 (2010)
Gorup, L.F., Longo, E., Leite, E.R., Emerson, R.: Moderating effect of ammonia on particle growth and stability of quasi-monodisperse silver nanoparticles synthesized by the Turkevich method. J. Colloid Interface Sci. 360, 355–385 (2011)
Sahni, G., Gopinath, P., Jeevanandam, P.: A novel thermal decomposition approach to synthesize hydroxyapatite-silver nanocomposites and their antibacterial action against GFP-expressing antibiotic resistant E. coli. Colloids Surf. B 103, 441–447 (2013)
Lee, W.M., An, Y.J., Yoon, H., Kweon, H.S.: Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water-insoluble nanoparticles. Environ. Toxicol. Chem. 27(9), 1915–1921 (2008)
Henglein, A.: Physicochemical properties of small metal particles in solution: “microelectrode” reactions, chemisorption, composite metal particles, and the atom-to-metal transition. J. Phys. Chem. 97(21), 5457–5471 (1993)
Ahmad, A., Mukherjee, P., Senapati, P., Mandal, D., Islam Khan, M., Kumar, R., Sastry, M.: Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B 28, 313–318 (2003)
Sastry, M., Patil, V., Sainkar, S.R.: Electrostatically controlled diffusion of carboxylic acid derivatized silver colloidal particles in thermally evaporated fatty amine films. J. Phys. Chem. B 102, 1404–1410 (1998)
Duran, N, Marcato, PD, Alves, OL, De Souza, CIH, Esposito, E.: Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J. Nanobiotechnol. 3 (2005). doi:10.1186/1477-3155r-r3-8
Zhou, Y., Lin, W., Huang, J., Wang, W., Gao, Y., Lin, L., Li, Q., Du, M.: Biosynthesis of gold nanoparticles by foliar broths: roles of biocompounds and other attributes of the extracts. Nanoscale Res. Lett. 5, 1351–1359 (2010)
Slimestad, R., Fossen, T., Vågen, I.M.: Onions: a source of unique dietary flavonoids. J. Agric. Food Chem. 55, 10067–10080 (2007)
Miean, K.H., Mohamed, S.: Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 49, 3106–3112 (2001)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Sahni, G., Panwar, A. & Kaur, B. Controlled green synthesis of silver nanoparticles by Allium cepa and Musa acuminata with strong antimicrobial activity. Int Nano Lett 5, 93–100 (2015). https://doi.org/10.1007/s40089-015-0142-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-015-0142-y