Abstract
The growth rate of farmed fish is an important factor regarding aquaculture success. An understanding of the cellular events that occur in skeletal muscle when fish undergo periods of fasting and refeeding provides information useful in developing alternative feeding strategies for improving muscle growth in commercially cultivated species. To evaluate the effect of 1–3 weeks of fasting and 10 weeks of refeeding in Nile tilapia juveniles, we analyzed the growth performance and changes in muscle cellularity and the expression of the following growth and muscle related genes: MyoD, myogenin, IGF-1, IGF-1 receptor, MuRF-1, atrogin-1 and myostatin. Reduced body mass was observed in all three groups of fasted fish during their time off feed, and 10 weeks of refeeding resulted in partial compensatory growth of body mass. No differences in the frequency of white muscle fiber diameters were observed between fasted and fed control fish treatments. However, changes in gene expression induced by fasting and refeeding were found. IGF-1 receptor, ubiquitin ligases MuRF1 and atrogin-1 expression increased during the 1–3 weeks of fasting, while IGF-1 levels dropped significantly (P < 0.001) compared to the control treatment. Furthermore, myogenin mRNA level in fish submitted to 3 weeks of fasting was higher in comparison to the control treatment (P < 0.05). Overall, our results showed that 1–3 weeks of fasting can induce muscle atrophy activation in Nile tilapia juveniles, and 10 weeks of refeeding is enough to induce only partial compensatory growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fasting is a phenomenon that can occur naturally in the environment due to low food supply, during fish migration, overwintering or during reproduction, throughout which fish demonstrate the ability to survive without food for lengthy periods (Love 1970; Bower et al. 2009). After a period of growth depression and when favorable conditions are restored, there occurs a phase of accelerated growth, called compensatory growth (Ali et al. 2003). Partial, full and over-compensation growth have all been evoked in fish. However, over-compensation has only been observed when cycles of deprivation and satiation feedings have occurred. Compensatory growth has been partially linked to hyperphagia when rates of food consumption are significantly higher than those in fish that have not experienced a previous growth depression episode. The severity of the growth depression increases the duration of the hyperphagic phase rather than maximum daily feeding rate. In some studies, growth efficiencies were notably greater during the compensatory growth stage (Ali et al. 2003; Dobson and Holmes 1984). In teleosts, the maintenance of skeletal muscle development and growth is a complex process, and may be influenced by environmental factors and the nutritional and physiological states of the animal (Johnston et al. 2008, 2011). A significant amount of research effort has been expended in understanding the regulatory factors surrounding muscle accretion, specifically evaluation of the genes involved. Myogenic regulatory factors (MRFs) are a family of four transcription factors (MyoD, myogenin, myf5 and MRF4/myf6) that are highly conserved in vertebrates and are responsible for the control of myogenesis and muscle growth. Each gene has evolved to regulate a specific area of myogenesis. For instance, MyoD and myf5 are essential regulators of muscle cell proliferation determination, whereas myogenin and myf4 act as regulators of muscle cell differentiation (Rudnicki et al. 1993; Steinbacher et al. 2007; Johnston et al. 2008).
Muscle growth is also controlled by the regulator myostatin. Myostatin is a member of the transforming growth factor-β (TGF-β) superfamily that functions as a negative regulator of skeletal muscle development and growth in mammals; however, in fish, myostatin is also present in other tissues and in other forms (Maccatrozzo et al. 2001; Rios et al. 2002; Acosta et al. 2005; De Santis et al. 2012). Another regulator of muscle growth in vertebrates is the insulin-like growth factor-1 (IGF-1) which represents the main autocrine and paracrine regulatory mechanisms of skeletal muscle by activation of the PI3 k/Akt pathway which consequently increases protein synthesis, associated with muscle hypertrophy (Glass 2003; Sacheck et al. 2004; Kandarian and Jackman 2006; Fuentes et al. 2013).
Muscle protein degradation is regulated by several processes, one of which is the ubiquitin–proteasome pathway. Two members of these muscle-specific ubiquitin ligases are the muscle RING finger 1 (MuRF1) and muscle atrophy F-box (MAFbx/atrogin-1). Studies show that an increase in MuRF1 and atrogin-1 expression causes fiber reduction due to catabolic conditions (Glass 2005).
The Nile tilapia Oreochromis niloticus is globally one of the most important farmed finfish species and has been found to effectively adapt to a wide range of rearing conditions (Abdel-Hakim et al. 2009; Fox et al. 2009). However, tilapia culture is affected by water shortage, low food supply in floating cages, prolonged winter, fish reproductive period and other conditions. Understanding the molecular mechanisms that control muscle growth in tilapia would be beneficial for improving production under different conditions that occur such as short-term fasting.
Previously we showed that short periods of fasting promoted muscle cell fiber atrophy in small juvenile tilapia (0.6 g) and that refeeding caused compensatory mass gain correlated with increased expression of muscle growth related genes (MyoD, myogenin and myostatin) (Nebo et al. 2013). Thus, the aim of this study was to evaluate the effects in larger juveniles (25–30 g) under longer fasting and refeeding time intervals to test the hypothesis that the ubiquitin–proteasome pathway is activated in tilapia juveniles during periods of fasting, and that IGF-1 is overexpressed during the refeeding to induce compensatory growth.
Materials and methods
Ethical procedures
The study was approved by the Comitê de Ética no Uso de Animais (CEUA) of the Faculdade de Ciências Agrárias e Veterinárias, UNESP Univ Estadual Paulista (CEUA/FCAV/UNESP, 009436/11).
Experimental design
Fish were obtained from a commercial farm (Royal Fish, Itupeva—São Paulo, Brazil) and acclimated (15 days) to the experimental conditions at the Laboratório de Nutrição de Organismos Aquáticos, of the Centro de Aquicultura. A total of 1120 fish were stocked in thirty-two 150-L tanks (35 fish/tank) with constant water flow and aeration. Nile tilapia Oreochromis niloticus juveniles, Chitralada–Thai strain were weighed (initial weight 30.2 ± 0.9 g) and randomly distributed into four experimental treatments with eight replicates: (FC) control, fish were fed continuously during the 13 weeks; (F1) 1 week of fasting (1WF) and 10 weeks of refeeding (10WR), (F2) 2 weeks of fasting (2WF) and 10WR; (F3) 3 weeks of fasting (3WF) and 10WR. The experiment lasted 13 weeks, and a fixed period of 10 weeks of refeeding was maintained for all fasting treatments. When not fasted, fish were fed to apparent satiation with an extruded commercial diet (32% crude protein and 6.5% lipid) three times per day (9 am, 2 pm, and 5 pm).
White muscle samples (n = 8 per treatment) were collected to analyze morphology, morphometry and the expression of growth related and muscle atrophy genes, at the end of each period of fasting (1WF, 2WF and 3WF) and after 10WR. Water variables were monitored weekly and maintained at: temperature 30.1 ± 0.32 °C, pH 7.89 ± 0.14 and dissolved oxygen 4.74 ± 0.86 mg/L. For sampling, fish were anesthetized (0.1 g/L of benzocaine) and individually weighed and measured. The body mass (g) was measured at the beginning of the experiment (0 day) and after each period of fasting: one (1WF), two (2WF) and three (3WF) weeks, and after one (1WR), two (2WR), six (6WR) and 10 weeks of refeeding (10WR). A graphical representation of the experimental design is provided in Supplementary file 1.
Morphology and morphometry of muscle fibers
Fish were euthanized with benzocaine (0.4 g/L) and white muscle samples (n = 8 fish/treatment) were collected from the dorsal region, fixed in Karnovsky solution (8% paraformaldehyde and 2.5% glutaraldehyde in PBS—phosphate buffer saline) and embedded in Historesin® (Leica, Germany). Histological transverse sections (4 µm) were obtained and stained with hematoxylin-phloxine B to analyze muscle fiber diameter. To estimate the degree of muscle hypertrophy, hyperplasia or atrophy the diameter of 700 white muscle fibers for each treatment (n = 8 per treatment) were measured from between three to four fields per sample. The fibers were binned into classes according to their diameter (d, µm): class 10 = d < 10, class 30 = 10 > d < 30, class 50 = 30 > d < 50 and class 60 = d ≥ 50, and white muscle fibers frequency in different diameter classes was determined. Images were captured with a Zeiss Axioplan 2 microscope and analyzed with Zeiss Axiovision 4.7 software (Carl Zeiss Microscope, LLC, USA). Morphology of muscle cells at different periods is provided in Fig. 1 and in color in Supplementary file 2.
Morphology of muscle cells after different periods of fasting and refeeding. Transverse section of white muscle fiber of Nile tilapia Oreochromis niloticus juveniles after 1 (1WF), 2 (2WF) and 3 (3WF) weeks of fasting and after 10 weeks of refeeding (10WR). a F1 at 1WF; b F1 at 10WR; c F2 at 2WF; d F2 at 10WR; e F3 at 3WF; f F3 at 10WR. Small fibers around big fibers, forming a growth mosaic pattern. Central nucleus (CN), peripheral nucleus (PN), perimysium (P) and endomysium (E). (FC) control, fed continuously during the 13 weeks; (F1) 1 week of fasting and 10WR, (F2) 2 weeks of fasting and 10WR; (F3) 3 weeks of fasting and 10WR. Hematoxylin–phloxine stain. Scale bars: 20 µm, 40 X
Gene expression
White muscle samples (n = 8) were collected from the dorsal region of fish at the beginning of the experiment and at 1WF, 2WF, 3WF and after 10WR during the experiment and frozen in liquid nitrogen. Total RNA was extracted using TRIzol® Reagent according to the manufacturer’s protocol (Life Technologies, Carlsbad, CA, USA). Extracted RNA integrity was confirmed by electrophoresis on a 1% agarose gel stained with GelRed (Biotium, Hayward, CA, USA). Quantity of extracted RNA was determined using a NanoVue™ Plus Spectrophotometer (GE Healthcare, Piscataway, NJ, USA). RNA purity was ensured by obtaining a 260/280 nm OD ratio ≥ 1.8. Total RNA were solubilized in RNase-free water and incubated with Dnase I (Life Technologies, Carlsbad, CA, USA) to remove any DNA present in the samples. For expression analysis using SYBR, total RNA (2 μg) was reverse transcribed using the High Capacity cDNA archive kit with RNase inhibitor (Life Technologies, Carlsbad, CA, USA), according to the manufacturer’s instructions. At the end of the reaction, the samples were stored at − 20 °C.
Gene expression analysis was evaluated by reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR). For each gene, specific primers were tested at different concentration levels to determine optimal amplification. Samples of cDNA were amplified with specific primers for O. niloticus MyoD, myogenin, myostatin (Nebo et al., 2013), IGF-1, IGF-1 receptor, and the ubiquitin regulating gene, MAFbx/atrogin-1 designed from cDNA nucleotide sequences, available in GenBank (http://www.ncbi.nlm.nih.gov/pubmed/nucleotide). The gene expression analyses were performed with an ABI 7500 Fast Real-Time PCR System (Life Technologies, Carlsbad, CA, USA). The reactions were run in duplicate using Fast SYBR Green Master Mix (Life Technologies, Carlsbad, CA, USA), according to the manufacturer’s instructions. Target gene expression was normalized to ubiquitin-like protein 3 (ubl3) gene expression and melting dissociation curves were performed to confirm that only a single product was amplified. Control reactions lacking cDNA template were also included to check for reagent contamination. The normalization genes were previously tested and the gene with the least variation between samples was chosen.
For the analysis of MuRF1, atrogin-1 and beta-actin genes expression primers and probes were utilized. Reactions were run on an ABI Prism 7900HT Sequence Detection System (Life Technologies, Carlsbad, CA, USA) using Verso 1-Step RT-qPCR ROX Mix (Thermo Fisher Scientific, Waltham, MA, USA). The reactions were run in duplicate using a TaqMan Assay kit (Life Technologies, Carlsbad, CA, USA), according to the manufacturer’s instructions. Samples were normalized to beta-actin gene expression. Relative gene expression was calculated using the (2−ΔΔCT) method using the FC group as the untreated sample (Livak and Schmittgen 2001). For IGF-1, IGF-1 receptor, beta-actin, MuRF1, and atrogin-1 genes primers and probes were designed using PrimerQuest (Integrated DNA Technologies, Coralville, IA) (Table 1).
Statistical analysis
Morphometric and gene expression data were analyzed by one-way ANOVA for differences between means, and where significant (P < 0.05) post hoc analyses tested with Tukey’s, using the Statistical Analysis System SAS V9 software (SAS Institute Inc., Cary, NC, USA). Two comparisons were made: fed control (FC) between fasted treatments (F1, F2, and F3); and also fasted treatments were compared between groups after fasting (1WF, 2WF, and 3WF) and 10 weeks of refeeding. All data were tested for homogeneity of variance using Brown–Forsythe test (P < 0.05) and normality using Cramer–von Mises test (P < 0.05). Logarithmic transformations (natural logarithm) were used, in analysis of body mass, IGF-1, IGF-1 receptor, myogenin, atrogin-1 and MuRF1, as relevant correction of original data.
Results
Body mass response to fasting and refeeding
Survival rate of Nile tilapia juveniles was greater than 83% in the FC (83.9%) F1 (86.1%), and F2 (87.5%) treatments, whereas in the F3 it was much lower (79%). However, these differences were not significant.
As expected, no body mass (BM) increase was observed during the fasting period (week 1–week 3); On the other hand, fasted fish lost significant weight during fasting in relation to FC (P < 0.001) (Table 2). BM was increased during the refeeding periods (1WR, 2WR, 6WR and 10WR), but it was lower in all fasted fish compared to FC group (P < 0.001). Regarding the 1WR treatment, the BM in fish from F1 and F2 was significantly higher compared to the F3 treatment. Similar results were observed for the treatment group 2WR, the BM was higher in fish from F1 and F2 treatments compared to F3. However, at 6WR, the BM increased in fish from F3 compared to F1 and F2 treatments. After 10WR, the BM in fish from F1 was significantly higher compared to the F2 and F3 treatments (Table 2).
Morphological and morphometric analyses
Muscle morphology analysis revealed that at 1WF the F1 treatments showed a high number of muscle fibers with a central nucleus, exhibiting a pattern of mosaic hyperplasia (Table 3). Nonetheless, the muscle fiber morphology at 2WF or 3WF in groups of fish from F2 and F3 treatments was different from what was observed the week before, showing an increased number of mature muscle fibers with multinucleated cells with peripheral nuclei (Table 3). After 10WR, in the fasted treatments (F1, F2 and F3), muscle fiber morphology exhibited a typical mosaic pattern with polygonal shaped fibers, small fibers adjacent to big fibers (Table 3).
After 1WF, 2WF and 3WF, morphometric analyses showed a higher quantity (> 95%) of smaller muscle fibers in between classes 10 and 30, and a lower quantity (< 0.6%) in class 50. The frequency of muscle fibers in diameter classes did not differ significantly between fasted fish and the respective FC group (Table 3). However, after 10WR the distribution of white muscle fiber frequency in diameter classes changed in fish from F1, F2, and F3 treatments, showing higher numbers of big fibers in classes 50 and 60 compared to classes 10 and 30. Similar to the fasting period, no differences were found among FC and the fasted fish, after 10WR (Table 3).
Increased expression of atrogenes and IGF-1 reduction during fasting
The IGF-1 gene expression, at 1WF, 2WF and 3WF, was downregulated, exhibiting significant differences (P < 0.028) in comparison to the FC group; and after 10WR, the IGF-1 levels were increased, being similar to the IGF-1 mRNA level in the FC group, showing a substantial response to refeeding. Furthermore, after 10WR, the F1 group was found to express IGF-1 significantly higher compared to the F2 group (P < 0.017) (Fig. 2a). Conversely, the IGF-1 receptor was upregulated in the fish from the F1, F2 and F3 groups during the fasting periods, but after 10WR, only the expression of IGF-1 receptor in the F3 group was found to be significantly reduced (P < 0.03) in comparison to the FC group (Fig. 2b).
Relative gene expression of IGF-1, IGF-1 receptor, MyoD and myogenin in white skeletal muscle of Nile tilapia Oreochromis niloticus juveniles subjected to fasting and refeeding. Gene expression was evaluated after 1 (1WF), 2 (2WF) and 3 (3WF) weeks of fasting and after 10 weeks of refeeding (10WR). a Relative expression of IGF-1; b relative expression of IGF-1 receptor; c relative expression of MyoD; d relative expression of myogenin. (FC) control, feeding continuously during the 13 weeks; (F1) 1 week of fasting and 10WR; (F2) 2 weeks of fasting and 10WR; (F3) 3 weeks of fasting and 10WR. Uppercase letters denote significant differences between F1, F2, and F3 treatments after fasting and refeeding, and *significant difference (P < 0.05) between control and fasted treatments. Data are mean ± SEM (n = 8)
MyoD gene expression was found to not differ among all the groups, either in the fasting or the refeeding group (Fig. 2c). Regarding myogenin expression, its mRNA levels were not found to differ significantly except at 3WF where they were higher compared to the FC group (Fig. 2d).
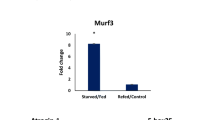
The myostatin mRNA levels, after fasting and refeeding, did not differ between control and fasted groups (Fig. 3a). The atrogin-1 and MuRF-1 gene expression levels, at 1WF, 2WF and 3WF, increased significantly (P < 0.004) compared to the FC group. However, after 10WR, the atrogin-1 and MuRF-1 levels in all fasted groups were similar to the FC (Fig. 3b, c). Atrogin-1 expression was serially higher after 10WR and significantly different between the F1, F2 and F3 groups, but not significantly different compared to the control (Fig. 3b).
Relative gene expression of myostatin, atrogin-1 and MuRF-1 in white skeletal muscle of Nile tilapia Oreochromis niloticus juveniles subjected to fasting and refeeding. The gene expression were calculated after 1 (WF), 2 (2WF) and 3 (3WF) weeks of fasting and after 10 weeks of refeeding (10WR). a Relative expression of myostatin; b relative expression of atrogin-1; c relative expression of MuRF1. (FC) control, fed continuously during the 13 weeks; (F1) 1 week of fasting and 10 weeks of refeeding; (F2) 2 weeks of fasting and 10WR; (F3) 3 weeks of fasting and 10WR. Uppercase letters denote significant differences between F1, F2, and F3 treatments after fasting and refeeding, and *significant difference (P < 0.05) between control and fasted treatments. Data are mean ± SEM (n = 8)
Discussion
In the present work, we investigated if fasting for a certain interval followed by 10 weeks of refeeding would change muscle gene expression. Concurrently, we also wanted to evaluate if this starve/refeed regime would cause alterations in white muscle fiber plasticity and morphology in Nile tilapia Oreochromis niloticus juveniles.
The periods of fasting evaluated, 1, 2 and 3 weeks (1WF, 2WF, and 3WF, respectively) were not sufficient to cause any significant decrease in weight in fasted tilapia juveniles during the fasting period. However, significant differences were detected between the groups during the refeeding periods. The inability of the fasted groups to achieve body mass as the FC fish after refeeding, as previously reported, could be that the fasting periods were not of sufficient to induce a strong compensatory growth response in these animals at this phase of development (Wang et al. 2000).
To understand if fasting periods were detrimental to fish skeletal muscle growth and to determine the response of fish refeeding after 10 weeks, muscle tissue was analyzed by evaluating the morphology and the diameters of muscle fibers to determine the relative degree of hypertrophy, hyperplasia or atrophy of fibers. High frequency (over 90%) of small white muscle fibers in classes 10 and 30 during one 1WF, two 2WF and three 3WF weeks, in all treatments, showed that muscle hyperplasia was occurring in the juvenile tilapia within this study. Hyperplasia was evident within the first week of the experiment by the observation of several small fibers with central nuclei. This morphological feature characterizes skeletal muscle development in mosaic hyperplasia, with small fibers containing central nuclei surrounding big fibers throughout the myotome (Johnston et al. 2003; Johnston and Hall 2004; Johnston et al. 2008; Steinbacher et al. 2007; Valente et al. 2013). However, after 10 weeks of refeeding the growth pattern changed, with increasing the frequencies of muscle fibers in the > 50 µm diameter classes and decreasing the frequencies in the 10 and 30 diameter classes. This change in fiber distribution was demonstrated by the fish in all the fasted and fed groups and can be considered as evidence of hypertrophic muscle growth (Veggeti et al. 1993; Valente et al. 1999; Zimmerman and Lowery 1999; Johnston 2006).
IGF-1 mRNA levels dropped significantly throughout periods of fasting (1WF, 2WF, and 3WF), but after subsequent refeeding, the IGF-1 muscle expression in previously fasted fish was identical to the FC group. Among fasting treatments, the mRNA levels were higher in the F1 group. These findings are in agreement with other studies in which reductions in muscle IGF-1 levels as a result of fasting, and subsequent recovery following 2–4 weeks of refeeding have also been seen in studies with tilapia juveniles Oreochromis mossambicus (Fox et al. 2009), rainbow trout Oncorhynchus mykiss fry (Montserrat et al. 2007), and rainbow trout adults submitted to 10 weeks of food deprivation and 4–34 days of refeeding (Chauvigne et al. 2003).
In channel catfish, Ictalurus punctatus the IGF-1 expression decreased after 30 days of fasting, although after refeeding, its expression level was found to be similar to the fed control group (Peterson et al. 2009). In rainbow trout treated with IGF-1 during feed deprivation, inhibition of atrogin-1 expression was observed, along with a reduction of proteolytic activity (Cleveland et al. 2009). In the present study, IGF-1 receptor expression was upregulated significantly during 1–3 weeks of fasting in F1, F2, and F3, compared to FC. The high level of IGF-1 receptor in juvenile tilapias, during fasting periods, appears to correlate with a downregulation of IGF-1 expression.
Other studies reported diverse results with different fish species and experimental conditions, and in general, fasting causes significant changes in MRF gene expression (Chauvigne et al. 2003; Valente et al. 2012). However, our results showed that there was no change in the expression of MyoD between FC and the fasted groups F1, F2 and F3 during the entire experimental period. Similar results were found in rainbow trout, where no significant change in MyoD expression levels was found in fish in response to starvation and refeeding (Johansen and Overturf 2006). The same results were also noted in another study with rainbow trout fasted for 1, 2 and 4 weeks, where MyoD levels in white muscle were not significantly affected by fasting (Montserrat et al. 2007). Furthermore, myogenin levels in Nile tilapia juveniles measured at the first 2 weeks of fasting (2WF) and after 10 weeks of refeeding (10WR) did not differ in relation to FC. In gilthead sea bream, myogenin gene expression was also found to not be affected after 30 days of food deprivation (Garcia et al. 2014). MyoD expression can be expected to be reduced in fasting fish as the lack of available nutrient energy would preclude muscle development and growth.
If the expression of myostatin was high, it would explain the relatively low expression of MyoD in fasted treatments. However, in our study, skeletal muscle myostatin levels were not different in Nile tilapia juveniles between fasted and control fish throughout the experiment. A long-term study of fasting in adult tilapia also showed that myostatin expression was unaffected by fasting (Rodgers et al. 2003) Our findings suggest that the level of starvation in these juveniles was enough to activate an alternative mechanism for regulating muscle growth rather than through myostatin. In rainbow trout deprived of food for 10 weeks and refeed for 34 days, the myostatin expression, similar to our findings, was not significantly changed during the experiment (Chauvigne et al. 2003). However, in tilapia fingerlings (0.6 g), myostatin expression was upregulated after 5 days of fasting in comparison to the fed control; nonetheless, after refeeding no differences were detected (Nebo et al. 2013). Likewise in another study performed with tilapia in larval phase, myostatin mRNA levels were upregulated by fasting and then reduced during refeeding (Rodgers et al. 2003). Taken into consideration the above, these results suggest that the degree of catabolism induced by fasting in tilapia is greater in larvae and fingerlings, likely due to lower body reserves, than in juvenile and adult fish that have more energy storage, and thus a higher capacity to tolerate fasting conditions.
The levels of atrogin-1 and MuRF1 expression in white skeletal muscle after 1–3 weeks of fasting increased rapidly, suggesting an increase in muscle turnover. However, the expression of atrogin-1 and MuRF1 after 10 weeks refeeding dropped to basal levels, and no significant differences were found among all groups (P > 0.05). Our results corroborate other reported findings with different fish species submitted to different fasting protocols. For instance, in rainbow trout juveniles, after 14 days of food deprivation the atrogin-1 levels increased and 12 h after refeeding decreased significantly (Seiliez et al. 2008); in salmon white muscle, the expression of MuRF1 increased in fasted fish compared to fed control (Tacchi and Bicherdike 2012); and in Atlantic salmon and zebrafish, atrogin-1 and MuRF1 were also found to be upregulated during fasting and downregulated after refeeding (Bower et al. 2009; Bower and Johnston 2010; Amaral and Johnston 2011).
It is well established in vertebrates that insulin-like growth factor-1 stimulates activation of the PI3 K/Akt/mTOR cascade, increasing protein synthesis and hypertrophic muscle growth (Engert et al. 1996; Zhang et al. 2008; Duan et al. 2010), and inactivates FOXO transcription factors, decreasing the expression of atrogin-1/MAFbx and MuRF1 (Sacheck et al. 2004; Edstron et al. 2006; Cleveland et al. 2009; Fuentes et al. 2013). In this context, the lower IGF-1 expression observed in fasted tilapia juveniles likely plays a role in the higher expression of MuRF1 and atrogin-1, consequently inducing muscle atrophy, over extended periods of time, and blocking somatic growth. On the other hand, after refeeding, the expression of IGF-1 increased to the basal level, equal to the control, resulting in growth recovery.
In conclusion, the results from the present study demonstrated that the corresponding upregulation of atrogin-1 and MuRF1 and the drop in IGF-1 expression during fasting periods correlate with an initial stage of muscle atrophy. However, no signs of atrophy were observed in muscle morphology, but the expression of atrophy genes changed during fasting. Nevertheless, the increased IGF-1 levels after 10 weeks of refeeding presumably activated the Akt pathway, which correspondingly decreased the expression of genes involved in the ubiquitin–proteasome pathway, thus reducing rates of protein degradation and contributing to growth recovery. Furthermore, we observed partial compensatory growth (fish grew, but did not reach the same body mass than the control fish), after refeeding in Nile tilapia juveniles. These results contribute to a better understanding of molecular control related to skeletal muscle growth during fasting and after refeeding. This information will be beneficial in developing best management plans for feeding optimized to economically maximize growth during production specifically if phase feeding or fasting can be used.
References
Abdel-Hakim NF, Abo-State HA, Al-Azab AA, El-Kholy KHF (2009) Effect of feeding regimes on growth performance of juvenile hybrid tilapia (Oreochromis niloticus × Oreochromis aureus). World J Agric Sci 5:49–54
Acosta J, Carpio Y, Borroto I, González O, Estrada MP (2005) Myostatin gene silenced by RNAi show a zebrafish giant phenotype. J Biotechnol 119:324–331
Ali M, Nicieza A, Wootton RJ (2003) Compensatory growth in fishes: a response to growth depression. Fish Fish 4:147–190
Amaral IPG, Johnston IA (2011) Insulin-like growth factor (IGF) signaling and genome-wide transcriptional regulation in fast muscle of zebrafish following a single-satiating meal. J Exp Biol 214:2125–2139
Bower NI, Johnston IA (2010) Transcriptional regulation of the IGF signaling pathway by amino acids and insulin-like growth factors during myogenesis in Atlantic salmon. PLoS One 5:1–14
Bower NI, Taylor RG, Johnston IA (2009) Phasing of muscle gene expression with fasting-induced recovery growth in Atlantic salmon. Front Zool 6:1–13
Chauvigne F, Gabillard JC, Weil C, Rescan PY (2003) Effect of refeeding on IGF-1, IGF-II, IGF receptors, FGF2, FGF6, and myostatin mRNA expression in rainbow trout myotomal muscle. Gen Comp Endocrinol 132:209–215
Cleveland BM, Weber GM, Bleming KP, Silverstein JT (2009) Insulin-like growth factor-I and genetic effects on indexes of protein degradation in response to feed deprivation in rainbow trout (Oncorhynchus mykiss). Am J Physiol Regul Integr Comp Physiol 297:1332–1342
De Santis C, Gomes GB, Jerry DR (2012) Abundance of myostatin gene transcripts and their correlation with muscle hypertrophy during the development of barramundi, Lates calcarifer. Comp Biochem Physiol B 163:101–107
Dobson S, Holmes R (1984) Compensatory growth in the rainbow trout, Salmo gairdneri Richardson. J Fish Bio 25:649–656
Duan C, Ren H, Gao S (2010) Insulin-like growth factors (IGFs), IGF receptors and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen Comp Physiol 167:344–351
Edstron E, Altun M, Hagglund M, Ulfnake B (2006) Atrogin-1/MAFbx and MuRF1 are downregulated in aging-related loss of skeletal muscle. J Gerontol Ser Biol Sci Med Sci 61:663–674
Engert C, Berglund EB, Rosenthal N (1996) Proliferation precedes differentiation in IGF-I stimulated myogenesis. J Cell Biol 135:431–440
Fox BK, Breves JP, Hirano T, Grau EG (2009) Effects of short- and long-term fasting on plasma and stomach ghrelin, and the growth hormone/insulin-like growth factor I axis in the tilapia, Oreochromis mossambicus. Domest Anim Endocrinol 37:1–11
Fuentes EN, Valdes JA, Molina A, Bjornsson BT (2013) Regulation of skeletal muscle growth in fish by the growth hormone—insulin-like growth factor system. Gen Comp Endocrinol 192:136–148
Garcia de la Serrana D, Codina M, Capilla E, Jimenez-Amilburu V, Navarro I, Du SJ, Johnston IA, Gutierrez J (2014) Characterization and expression of myogenesis regulatory factors during in vitro myoblast development and in vivo fasting in the gilthead sea bream (Sparus aurata). Comp Biochem Physiol B 167:90–99
Glass DJ (2003) Molecular mechanisms modulating muscle mass. Trends Mol Med 9:344–350
Glass DJ (2005) Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37:1974–1984
Johansen KA, Overturf K (2006) Alterations in expression of genes associated with muscle metabolism and growth during nutritional restriction and refeeding in rainbow trout. Comp Biochem Physiol 144:119–127
Johnston IA (2006) Environment and plasticity of myogenesis in teleost fish. J Exp Biol 209:2249–2264
Johnston IA, Hall TE (2004) Mechanisms of muscle development and responses to temperature change in fish larvae. Am Fish Soc Symp 40:85–116
Johnston IA, Fernandez DA, Calvo J, Vieira VLA, North AW, Abercromby M, Garland T (2003) Reduction in muscle fibre number during the adaptive radiation of notothenioid fishes: a phylogenetic perspective. J Exp Biol 206:2595–2609
Johnston IA, Macqueen DJ, Watabe S (2008) Molecular biotechnology of development and growth in fish muscle Fisheries for global welfare and environment. 5th World Fish Congr., pp 241–262
Johnston IA, Bower NI, Macqueen DJ (2011) Growth and regulation of myotomal muscle mass in teleost fish. J Exp Biol 214:1617–1628
Kandarian SC, Jackman RW (2006) Intracellular signaling during skeletal muscle atrophy. Muscle Nerve 33:155–165
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−DDCT method. Methods 25:402–408
Love RM (1970) The chemical biology of fishes, vol I. Academic Press, New York
Maccatrozzo L, Bargelloni L, Rodaelli G, Mascarello F, Patarnelo T (2001) Characterization of the Myostatin gene in the gilthead sea bream (Sparus aurata): sequence, genomic structure and expression pattern. Mar Biotechnol 3:224–230
Montserrat N, Gabillard JC, Capilla E, Navarro MI, Gutiérrez J (2007) Role of insulin, insulin-like growth factors, and muscle regulatory factors in the compensatory growth of the trout (Oncorhynchus mykiss). Gen Comp Endocrinol 150:462–472
Nebo C, Portella MC, Carani FR, Almeida FLA, Padovani CR, Carvalho RF, Dal-Pai-Silva M (2013) Short periods of fasting followed by refeeding change the expression of muscle growth-related genes in juvenile Nile tilapia (Oreochromis niloticus). Comp Biochem Physiol B 164:268–274
Peterson BC, Small BC, Waldbieser GC, Bosworth BG (2009) Endocrine response of fast and slow-growing families of channel catfish. N Am J Aquac 70:240–250
Rios R, Carneiro I, Arce VM, Devesa J (2002) Myostatin is an inhibitor of myogenic differentiation. Am J Physiol Cell Physiol 282:993–999
Rodgers BD, Weber GM, Kelley KM, Levine MA (2003) Prolonged fasting and cortisol reduce myostatin mRNA levels in tilapia larvae; short-term fasting elevates. Am J Physiol Regul Integr Comp Physiol 284:1277–1286
Rudnicki MA, Schnegeisberg PNJ, Stead RH, Braum T, Arnold HH, Jaenish R (1993) MyoD or myf5 is required for the formation of skeletal muscle. Cell 75:1351–1359
Sacheck JM, Ohtsuka A, Mclary SC, Goldberg AL (2004) IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab 287:591–601
Seiliez I, Panserat S, Skiba-cassy S, Fricot A, Vachot C, Kaushik S, Tesseraud S (2008) Feeding status regulates the polyubiquitination step of the ubiquitin-proteasome-dependent proteolysis in rainbow trout (Oncorhynchus mykiss) muscle. J Nutr 138:487–491
Steinbacher P, Haslett JR, Obermayer A, Marschanllinger J, Bauer HC, Sanger AM, Stoiber W (2007) MyoD and Myogenin expression during myogenic phases in brown trout: a precocious onset of mosaic hyperplasia is a prerequisite for fast somatic growth. Dev Dyn 236:1106–1114
Tacchi L, Bicherdike R (2012) Muscle-specific RING finger (MuRF) cDNAs in Atlantic salmon (Salmo salar) and their role as regulators of muscle protein degradation. Mar Biotechnol 14:35–45
Valente LMP, Rocha E, Gomes EFS, Silva MW, Oliveira MH, Monteiro RAF, Fauconneau B (1999) Growth dynamics of White and red muscle fibers in fast and slow growing strains of rainbow trout. J Fish Biol 55:675–691
Valente LM, Bower NI, Johnston IA (2012) Postprandial expression of growth-related genes in Atlantic salmon (Salmo salar L.) juveniles fasted for 1 week and fed a single meal to satiation. Br J Nutr 108:2148–2157
Valente LM, Moutou KA, Conceição LEC, Engrola S, Fernandes JMO, Johnston IA (2013) What determines growth potential and juvenile quality of farmed fish species? Rev Aquac 5:5168–5193
Veggeti A, Mascarello F, Scapolo PA, Rowlerson MD, Carnevali CMD (1993) Muscle growth and myosin isoform transitions during development of a small teleost fish (Poecilia reticulate) (Atheriniformes, Poeciliidae): a histochemical, immunohistochemical, ultrastructural and morphometric study. Anat Embryol 187:353–361
Wang Y, Cui Y, Yang Y, Cai F (2000) Compensatory growth in hybrid tilapia, Oreochromis mossambicus × O niloticus, reared in seawater. Aquaculture 189:101–108
Zhang XD, Zhu YF, Cai LS, Wu TX (2008) Effects of fasting on the meat quality and antioxidant defenses of market-size farmed large yellow croaker (Pseudosciaena crocea). Aquaculture 280:136–139
Zimmerman AM, Lowery MS (1999) Hyperpastic development and hypertrophic growth of muscle fibers in the white seabass (Atractoscion nobilis). J Exp Zool 284:299–308
Acknowledgements
The authors are grateful to the Fundação de Amparo à Pesquisa do Estado de São Paulo/FAPESP for the financial support by means of scholarships (2011/08426-3 and 2013/13100-3) and a research grant (2011/22326-0) and to the facilities and team of the University of Idaho—Hagerman Fish Culture Experiment Station. We thank technician Karen Frank at Hagerman Fish Culture Experiment Station for helping in gene expression analyses.
Author information
Authors and Affiliations
Contributions
Caroline Nebo was involved in planning and carrying out the experiment, data analysis and writing the manuscript. Ken Overturf was involved in planning, data analysis and writing the manuscript. Andrezas Brezas was involved with data analysis and writing. Maeli Dal-Pai-Silva was involved with experimental design, data analysis and writing of the manuscript. Maria Celia Portell was involved with experimental design, data analysis and manuscript writing.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nebo, C., Overturf, K., Brezas, A. et al. Alteration in expression of atrogenes and IGF-1 induced by fasting in Nile tilapia Oreochromis niloticus juveniles. Int Aquat Res 9, 361–372 (2017). https://doi.org/10.1007/s40071-017-0182-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40071-017-0182-1