Abstract
The current study investigated how different fasting and refeeding regimes would impact Nile tilapia growth performance, histopathological examination, and gene expression of myostatin, myogenin, GH, IGF-1, and NPYa. Nile tilapia fish (n = 120) were randomly allocated into four groups, including the control group fed on a basal diet for 6 weeks (F6), group A starved for 1 week and then refed for 5 weeks (S1F5), group B starved for 2 weeks and then refed for 4 weeks (S2F4), while group C starved for 4 weeks and then refed for 2 weeks (S4F2). Fasting provoked a decrease in body weight coincided with more extended starvation periods. Also, it induced muscle and liver histological alterations; the severity was correlated with the length of fasting periods. Gene expression levels of GH, MSTN, MYOG, and NPYa were significantly increased, while IGF1 was markedly depressed in fasted fish compared to the control group. Interestingly, refeeding after well-planned short fasting period (S1F5) modulated the histopathological alterations. To some extent, these changes were restored after refeeding. Restored IGF-I and opposing fasting expression profiles of the genes mentioned above thus recovered weights almost like the control group and achieved satisfactory growth compensation. Conversely, refeeding following more extended fasting periods failed to restore body weight. In conclusion, refeeding after fasting can induce a compensatory response. Still, the restoration capacity is dependent on the length of fasting and refeeding periods through exhibiting differential morphological structure and expressions pattern for muscle and growth-related genes.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the aquaculture sector, growth achievement is an essential concern; therefore, feeding is necessary (Assan et al. 2021). Under normal feeding conditions, fish can grow and store energy reserves; however, body stores are assembled during feed restrictions to maintain life requirements (Zhang et al. 2008). Both fasting and refeeding regimes can be very informative in basic and applied research. During fasting, metabolism is switched to a catabolic condition inducing a low growth rate, but refeeding changes the situation approaching a hyper-anabolic status. Thus, fish attempt to enhance their growth rate (Nebo et al. 2013). Several neural pathways are involved in the regulation of food intake as well as energy metabolism in vertebrates. In the brain, the hypothalamus promotes central and peripheral signals that either enhance or suppress appetite into a unified physiological and behavioral response (Volkoff 2016; Rønnestad et al. 2017; Soengas et al. 2018). Neuropeptide Y (NPYa) is one of the signaling molecules involved, which plays a functional role in regulating energy homeostasis and appetite invertebrates including teleosts (Volkoff 2016; Volkoff et al. 2000; Rønnestad et al. 2017; Soengas et al. 2018). After growth depression during fasting periods and when restoration of favorable conditions is resumed, a compensatory growth will be achieved with growth acceleration higher than the growth rate of the control fish, which may be due to improved feed intake, mitogen production, as well as feed conversion efficiency (Ali et al. 2003; Rescan et al. 2017; Won and Borski 2013). Fish have shown partial, complete, and over-compensation in growth (Nebo et al. 2017). The compensatory growth response has been proposed to increase finfish aquaculture productivity practically. Fish can deal with fasting as a normal phenomenon in their lifetime due to seasonal fluctuations of poor food availability and migration trips. Even under cultural conditions, several fish can undergo starving periods during stressful situations, changes in water quality, or disease outbreaks (Barcellos et al. 2010; Sridee and Boonanuntanasarn 2012; Najafi et al. 2015; Yang et al. 2019). Skeletal muscle represents 40–60% of the total body mass in most studied fish (Weatherley and Gill 1985) and principally represents white muscle, the edible part of the fish (Zhang et al. 1996; Sänger and Stoiber 2001). Fasting and refeeding affect the muscle, one of the most critical tissues for aquaculture. Gene expression changes caused by starvation and refeeding affect muscle metabolism and growth rate and can sometimes impair muscle growth (Hornick et al. 2000; Hagen et al. 2009). In teleosts, the preservation of skeletal muscle development and growth depends mainly on nutrient availability, which modulates the GH/IGF1 axis (Reinecke et al. 2005; Liu et al. 2020) that regulates the metabolism of different nutrients, synthesis of protein in muscle, and other tissue growth (Butler and Le Roith 2001). The endocrine activity of GH includes two major pathways: the direct one mediated by binding of GH to its receptor (GHR) and the indirect pathway via induction of IGF-1 secretion that promotes different biological activities (Canosa et al. 2007). IGF-I represents the main autocrine and paracrine mitogenic poly-peptide which is considered the most promising candidate as an indicator for fish growth as it increases protein synthesis and is associated with muscle hypertrophy (Kandarian and Jackman 2006; Fuentes et al. 2003).
Several genes are also implicated in the regulation of muscle growth, including myostatin (MSTN) and myogenic regulatory factors (MRFs). MSTN belongs to the transforming growth factor-b (TGF-b) superfamily, which functions as a negative regulator for the growth of skeletal muscle that is highly conserved across vertebrate species, referring to its essential function in muscular development and growth regulation (Acosta et al. 2005; De Santis et al. 2012; Lv et al. 2016; Kang et al. 2017). Myogenic regulatory factors (MRFs) are a family that consists of four transcription factors (myogenin, MyoD, myf5, and MRF4/myf6) that regulate myogenesis and muscle growth. Each gene has evolved to control a specific area of myogenesis regulation. Zhao et al. (2019) stated that any other MRFs could not compensate MYOG function.
It is essential to notice that short-term starving can be one of the feeding approaches used to manage water quality issues and lessen the impacts of temperature fluctuation, pre-harvesting, and handling (Davis and Gaylord 2011); reduce disease-related mortality; or save the feed from boosting farm earnings (Gaylord and Gatlin 2001).
The Nile tilapia (O. niloticus) is one of the most worldwide important farmed finfish species that can effectively adapt to a wide range of rearing conditions (Fox et al. 2009; Abdo et al. 2021). However, information concerning the biological and molecular mechanisms of modulating tilapia growth remains limited. A better understanding of growth regulatory genes and their impacts on fish during fasting and refeeding could help determine the best regime to regulate and enhance their growth and development, which could be beneficial for fish farming. Therefore, we aimed to investigate how different fasting and subsequent refeeding regimes would affect fish growth by measuring the tilapia growth performance, liver and muscle histopathological findings, and the expression of some muscle growth-related genes.
Materials and methods
Ethical statement
All the experimental procedures were performed according to the Egyptian ethical codes for studies on experimental animals and approved by the Animal Ethical Committee, Kafrelsheikh University (Number: IAACUC-KSU-2020–22).
Fish, diet, and experimental design
A total of 120 healthy male mono sex Nile tilapia (Oreochromis niloticus) were collected from a private fish farm “El-Behaira Governorate, Egypt,” with an average initial body weight of 50 ± 12.4 g (mean ± SD) which were housed in the laboratory of Fish processing and Biotechnology Department, Faculty of Aquatic and Fisheries Sciences, Kafrelsheikh University in glass aquaria (40 × 60 × 80 cm) in a recirculating water system with mechanical filters. The fish were acclimated to laboratory conditions in glass aquaria for 14 days. They were then randomly distributed into 12 glass aquaria with ten fish per aquarium in triplicates for each of the four groups (30 fish per group), including the control group provided with a basal diet over the experiment 6 weeks (F6). Group (A) fasted for 1 week and refed for 4 weeks (S1F5), group (B) fasted for 2 weeks and refed for 4 weeks (S2F4), and group (C) fasted for 4 weeks and refed 2 weeks (S4F2). The experimental design is portrayed in Fig. 1. The fish were fed a basal diet prepared according to NRC (2011) (Table 1) that was provided twice daily (07:00 am and 05:00 pm) at a daily rate of 3% of their body weight (commercial diet manufactured by extrusion technology). All fish were weighed at the beginning of the experiment and every week until the end of the experiment (6 weeks). Water quality parameters were measured, including pH, dissolved oxygen, and ammonia. Fish waste and half of the aquarium water were siphoned daily and replaced with well-aerated and de-chlorinated water.
Tissue sampling
At the end of the fasting (1, 2, and 4 weeks) and the refeeding periods (5, 4, and 2 weeks) for each group, nine randomly sampled fish from each group were anesthetized using 150 mg/L MS222 (Argent Laboratories, Redmond, WA, USA). Tissue sections from the white muscle and liver were collected from each group and were divided into two parts; one part was kept in 2 mL sterile Eppendorf tubes and immediately shocked in liquid nitrogen for subsequent RNA extraction. At the same time, the other part was fixed in 10% neutral buffered formalin for histopathological examination.
Histopathological examination
The abdomen was dissected to obtain samples. Sections from the white muscle and liver tissues were collected from each group (9 fish /group) at the end of the fasting and at the end of the refeeding periods after being anesthetized using 150 mg/L MS222 (Argent Laboratories, Redmond, WA, USA). Samples were fixed in 10% neutral buffered formalin for 18–24 h. Afterward, they were dehydrated using ascending grades of ethanol (70–100%) and then treated with xylene and embedded in paraffin wax. Subsequently, 5-μm-thick sections were obtained with a rotatory microtome (Leica RM 2125), then stained with hematoxylin and eosin (H&E) stain, and examined with a light microscope (Leica DM 5000) (Bancroft and Gamble 2008).
RNA extraction and reverse-transcription polymerase chain reaction
RNA extraction from all samples was performed using TriZol reagent (iNtRON Biotechnology). The extracted RNA was examined via NanoDrop® BioDrop Spectrophotometer for A260 nm/A280 nm and concentration. For RNA reliability, all extracted RNA was electrophoresed on 1.5% denaturing agarose gel containing 0.5% ethidium bromide (Sigma, Germany) viewed under UV transilluminator (Azure c200).
cDNA synthesis and quantitative real-time PCR
Quantitative (Qrt-PCR) analysis of mRNA expression of Nile tilapia-specific primers for MSTN and MYOG in muscle samples and GH, IGFI, and NPYa in liver samples using β-actin as the reference gene was performed; primers’ sequences and accession numbers are shown in Table 2. Also, 2 μg of RNA from all triplicate samples was reverse transcribed using Maxime RT PreMix (Oligo dT primer) (iNtRON Biotechnology, Korea) following the manufacturer’s manual. The cDNAs were used as the template for RT-PCR using the SensiFast SYBR Lo-Rox kit (Bioline) in the MIC-PCR thermocycler® (Bio-molecular systems, Australia). The cycling conditions were as follows: activation at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 5 s, annealing at a specific temperature for each primer for 20 s, and extension at 72 °C for 20 s. All samples were tested in duplicates. At the end of each PCR thermal profile, a melt curve analysis was performed to determine amplification specificity at 72 to 95 °C at 0.3 °C per second. The obtained data were analyzed using the MIC-PCR software v 2.6.5. The relative differences in gene expression were calculated using threshold cycle (CT) values that were first normalized to those of the Nile tilapia (Oreochromis niloticus) β-actin reference gene and using ΔCT values as previously described by Pffafi (2001).
Statistical analysis
GraphPad Prism (GraphPad Software 9.0.1, San Diego, CA 92108, USA) was used for statistical analysis as follows.
-
Body weight (BW) curves: one-way ANOVA between groups in each time point separately, followed by Tukey’s multiple comparison test where P value < 0.05
-
Specific growth rate (SGR): one-way ANOVA between groups at each time point separately, followed by Tukey’s multiple comparison test where P value < 0.05. One-way ANOVA within-group at different time points in each time point followed by Tukey’s multiple comparison test where P value < 0.05
-
Protein efficacy ratio (PER) and feed conversion ratio (FCR): one-way ANOVA between groups at the end point of the 6 weeks trial followed by Tukey’s multiple comparison test where P value < 0.05
-
Differential gene expression: one-way ANOVA within group at different time points in each time point whereas the W0 is the control point, followed by Tukey’s multiple comparison test where P value < 0.05. One-way ANOVA between-group at each time point separately, whereas the F6 is the control group, followed by Tukey’s multiple comparison test where P value < 0.05
Results
Growth parameters
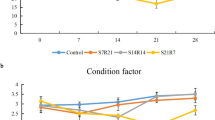
The growth indices during different fasting and refeeding periods are illustrated in Fig. 2. A short fasting period of 1 week significantly reduced the FBW (final body weight) and SGR with a non-significant change in PER and FCR in the control group. However, subsequent refeeding for 5 weeks achieved similar BW, SGR, PER, and FCR as the control group indicating total growth compensation. Moreover, more extended fasting periods for 2 or 4 weeks were significantly correlated to the BW and SGR reduction and FCR elevation than the control group. Moreover, subsequent refeeding following more extended fasting periods exhibited a reduced BW, SGR, and PER with elevated FCR than the control group indicating an inability to achieve growth compensation.
Effect of different experimental fasting and refeeding regimes on growth indices. All date are expressed as means ± SEM. Different lower cases indicate significant difference between different time points within the same group, while different upper cases indicate significant difference between different experimental groups within the same time point (P < 0.05). Body weight, BW; specific growth rate, SGR; protein efficacy ratio, PER; feed conversion ratio, FCR. n = 3 with 3 replicates per n
Histopathological examination
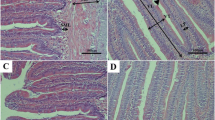
Histopathological investigation results of muscle and liver tissues of group A are presented in Fig. 3. The muscle of the control group reveals normal parallel multinucleated acidophilic rhabdomyolysis separated by endomysium of loose connective tissue (Fig. 3A). In contrast, the fasting group (1 week) showed a moderate increase in the thickness of perimysium, shrinkage of rhabdomyolysis, and interstitial edema of endomysium (Fig. 3B). However, the refeeding group (S1F5) decreases endomysium’s interstitial edema and signs of rhabdomyolysis regeneration (Fig. 3C). The hepatopancreas of fish of group A (S1F5), the control liver, reveals regular hepatic cords with intact hepatocytes containing centrally located nuclei and normal pancreatic acini surrounded by portal vein (Fig. 3D). The fasting group shows mild to moderate vacuolar degeneration of hepatocytes and shrinkage of pancreatic acinar cells surrounding the portal vein (Fig. 3E). The feeding group S1F5 showed regular hepatic cords with centrally located nuclei and normal pancreatic acini (Fig. 3F).
Photomicrograph of skeletal muscle and hepatopancreas of fish of group A (S1F5). A The control group (black arrows, normal parallel multinucleated acidophilic rhabdomyocytes; arrow heads, endomysium of loose connective tissue). B Fasting group (black arrow, perimysium; arrow head, rhabdomyocytes; white arrow, interstitial edema of endomysium). C Refeeding group (white arrow, interstitial edema of endomysium; black arrow, rhabdomyocytes). D The control group (black arrow, hepatic cords with intact hepatocytes containing centrally located nuclei; white arrow, pancreatic acini; arrow head, portal vein). E Fasting group (black arrow, hepatocytes; white arrow, shrinkage of pancreatic acinar cells; arrow head, portal vein). F Refeeding group (black arrow, hepatic cords with centrally located nuclei; white arrow, pancreatic acini). Stain H&E
Figure 4 represents the skeletal muscle of fish group B (S2F4). The control group showed multinucleated rhabdomyolysis separated by loose connective tissue of endomysium (Fig. 4A). The fasting group (2 weeks) reveals interstitial edema of endomysium, loss of striation of rhabdomyolysis, and presence of inflammatory cells along the course of blood vessels (Fig. 4B). Refeeding (S2F4) shows a decrease of interstitial edema, inflammatory cells in the endomysium, and signs of regeneration of rhabdomyolysis with the increase of acidophilia of muscle fibers (Fig. 4C). In the hepatopancreas of fish group B (Fig. 4D), the control group showed intact hepatocytes arranged in cords separated by blood sinusoids and radiating from a central vein (CV) (Fig. 4D). The fasting group (2 weeks) shows moderate degeneration of pancreatic acinar cells surrounded by the congested portal vein and moderate vacuolar degeneration of hepatocytes (Fig. 4E). The feeding group reveals mild hepatocytes’ vacuolar degeneration and pancreatic acinar cells’ mild degeneration surrounding congested portal vein (Fig. 4F).
Photomicrograph of skeletal muscle and hepatopancreas of fish of group B (S2F4). A The control group (black arrow, multinucleated rhabdomyocytes; arrow head, loose connective tissue of endomysium). B Fasting group (white arrow, interstitial edema of endomysium; black arrow, rhabdomyocytes; arrow head, inflammatory cells along the course of blood vessels). C Refeeding (white arrow, interstitial edema; arrow head, inflammatory cells in the endomysium; black arrow, rhabdomyocytes with the increase of acidophilia of muscle fibers). D The control group (black arrow, hepatocytes; arrow head, blood sinusoids; central vein (CV)). E the fasting group (white arrow, pancreatic acinar cells; arrow head, congested portal vein; black arrow, vacuolar degeneration of hepatocytes). F The feeding group (black arrow, hepatocytes; white arrow, pancreatic acinar cells; arrow head, congested portal vein). Stain H&E
Figure 5 shows the skeletal muscle of fish of group C (S4F2). The control group showed two types of muscle fibers: red muscle fibers and white muscle fibers (Fig. 5A). Refeeding group (S4F2) reveals moderate degeneration of pancreatic acinar cells and mild vacuolation of hepatocytes (Fig. 5F). The fasting group (4 weeks) shows shrinkage of rhabdomyolysis with a decrease of acidophilia, loss of striation, signs of degeneration (atrophy), and increased interstitial edema (Fig. 5B). The refeeding group (S4F2) shows normal acidophilia at the periphery of muscle fibers and decreases acidophilia in the center of muscle fibers and a hepatopancreas of fish group C: The control hepatocytes were intact with centrally located nuclei separated by blood sinusoids (Fig. 5D), while fasting caused necrosis of pancreatic acinar cells surrounded congested portal vein and vacuolar degeneration of hepatocytes (Fig. 5E).
Photomicrograph of skeletal muscle and hepatopancreas of fish of group C (S4F2). A The control group (black arrow, red muscle fibers; white arrow, white muscle fibers). B Fasting group (black arrow, shrinkage of rhabdomyocytes; white arrow, increased interstitial edema). C Refeeding group (black arrow, muscle fibers; white arrow, interstitial edema). D The control group (black arrow, intact hepatocytes with centrally located nuclei; arrow head, blood sinusoids). E Fasting group (white arrow, necrosis of pancreatic acinar cells; arrow head, congested portal vein; black arrow, hepatocytes). F Refeeding group (white arrow, pancreatic acinar cells; black arrow, mild vacuolation of hepatocytes). Stain H&E
Gene expression analysis
Our observations demonstrated that starvation and refeeding altered fish growth by modulating differential expression levels of the selected genes. Regarding the muscle expression levels of the MSTN gene, starvation significantly up-regulated its expression levels. The expression levels were correlated to the extension of the starvation period compared to the control group. However, refeeding reduced its expression levels (Fig. 6A) considerably.
Effect of different experimental fasting and refeeding regimes on the relative expression of A myostatin gene (MSTN) and B myogenin gene (MYOG) in muscle C growth hormone (GH) and D insulin-like growth factor-1 (IGF1) in the liver at different time points. All date are expressed as means ± SEM. Different lower cases indicate significant difference between different time points within the same group, while different upper cases indicate significant difference between different experimental groups within the same time point (P < 0.05). n = 3 with 3 replicates per n
Starvation-induced elevation of MYOG gene expression in the muscle is compared to the control group. However, refeeding significantly declined its expression level (Fig. 6B).
Concerning liver GH gene expression levels, starvation also significantly raised GH expression levels compared with the control group. However, after feeding restored, its expression levels were compared to the control group (Fig. 6C).
Interestingly, IGF1 gene expression levels were significantly depressed during starvation compared to the control group. However, expression levels were restored to the control level (Fig. 6D).
Regarding the liver expression levels of the NPYa gene, starvation significantly enhanced its expression levels in correlation to the length of starvation periods compared to the control group. However, its expression levels were declined considerably when refeeding is assumed (Fig. 7).
Effect of different experimental fasting and refeeding regimes on the relative expression of neuropeptide a (NPYa) in the liver at different time points. All date are expressed as means ± SEM. Different lower cases indicate significant difference between different time points within the same group, while different upper cases indicate significant difference between different experimental groups within the same time point (P < 0.05). n = 3 with 3 replicates per n
Discussion
The potential association between growth, histological muscle alterations, and some growth-related genes during fasting and subsequent refeeding remains unclear in Nile tilapia (Oreochromis niloticus L). The current research looked at the effects of various fasting and refeeding regimes on growth performance, muscle and liver histopathological changes, and some growth-related genes of Nile tilapia (Oreochromis niloticus L.) to find the best feeding regime that takes into account tilapia biology, appetite, and growth regulation for the best adaptability to environmental changes.
This study discovered that fasting reduced body weight and SGR considerably compared to the control group. Several researchers mentioned that white muscle fiber size is substantially decreased by fasting protocols, suggesting that muscle is the principal target in such conditions (Fauconneau et al. 1995; Martínez et al. 2002). In line with our findings, despite an average feed intake, tilapia growth was lowered by shorter cycles of feed restriction and feeding (Gallardo-Collí et al. 2020). This is most likely due to nutritional stress on the tilapia’s digestive capabilities, as the energy acquired from the meal was utilized less for growth (Sadoul and Vijayan 2016). In addition, Nile tilapia (O. niloticus) and hybrid tilapia (O. mossambicus × O. niloticus) exposed to feed restriction display weight loss because of deficiency of a maintenance ration (Gao and Lee 2012). Some researchers claim that feed restriction alters the biochemical composition of fish due to the usage of nutrients (protein and lipids) as energy sources (Cho 2005). The HSI and GSI of fish decreased due to a reduction in the number of meals they received. An increase in days of feed restriction may have exacerbated the decline. Therefore, it is probable that the fish utilized liver and gonadal energy stores to supplement their dietary needs during this period of restricted feeding (Jobling 1995).
In the same way, Liu et al. (2019) reported a decrease in the growing weight of fish subjected to feeding a low-protein diet (25%) once daily for 20 days. Because lipid is the predominant energy storage form in fish, the crude lipid content of muscle and viscera is significantly lower. In addition, Sakyi et al. (2021) reported that the growth performance measures of body weight, feed conversion ratio, viscerosomatic index, and condition factor all fell dramatically during starvation. Also, the activity of digestive enzymes was significantly lowered in the starved fish. Fats, glycogen, and proteins are degraded to maintain fish physiological homeostasis due to starvation, causing weight loss (Zheng et al. 2015). Furthermore, the feeding efficiency of restricted fish was decreased throughout the whole feeding process. Therefore, determining the ideal duration of deprivation or level of deprivation is critical (Gao et al. 2015).
The duration of feed deprivation determines the degree of compensatory development in fish (Wang et al. 2009). Some feedback mechanisms (e.g., changes in the relative sizes of organs/tissues and the chemical composition of tissues, organs, and the entire body) must respond appropriately for the animal to reach specific growth-related targets for compensatory growth to occur (Jobling 2010). When fish go through a fasting period, they usually use their bodily reserves to meet their energy demands (carbohydrates, lipid, and protein) (Favero et al. 2018).
Other experiments with tilapia have likewise found that lipids are mobilized as the predominant energy source during fasting (Nebo et al. 2018; Wang et al. 2009). In addition to the metabolism of liver glycogen for fuel, as reported by Hsieh and Shiau (2000), tilapia are physiologically better adaptable to prolonged feed restriction and feeding (Wasielesky et al. 2013).
The Nile tilapia likely supplemented their energy requirements throughout the feed limitation phase using liver and gonadal energy storage (Jobling 1995). During feed restriction periods in the tilapia, the mobilization of nutrients stored in the liver or gonads to supplement metabolic energy requirements affected organ size; this result suggests that body fat stores, such as muscle fat, would be more slowly mobilized to support metabolic energy requirements than glycogen (Torfi Mozanzadeh et al. 2017). Wang et al. (2009) reported that starved fish were shown to have higher SGR and feed intake during the refeeding period than a control group. This supported our result concerning the compensatory mechanism of fish. In addition, the most significant markers during compensating growth are increased feed intake and a low feed conversion ratio. Our study revealed that starvation affects growth, but it also affects skeletal muscle and liver histological architectures. Fasting for 1 week caused rhabdomyocyte shrinkage and endomysium interstitial edema in white muscle and modest to severe hepatocyte vacuolar degeneration and shrinking of pancreatic acinar cells surrounding the portal vein. Notably, the severity of the identified injuries was proportional to the length of fasting periods. Several studies reported that fasting caused liver degeneration, decreased hepatocyte size, and enlargement of the sinus in Chanos Chanos juveniles, Amphiprion melanopus, and green sturgeon (Acipenser medirostris) (Storch and Juario 1983; Green and McCormick 1999). Moreover, steatosis, inflammation, degeneration, necrosis, and hyperemia with reduced liver tissue were all observed in rainbow trout (Oncorhynchus mykiss) (Karatas et al. 2021), which could be related to impaired lipid transport, lipid biosynthesis, or both (Colakoglu and Donmez 2012), although the elevated blood flow, inflammation, necrosis, and enlargement of arterioles were all documented to be suggested reasons of hyperemia (Karataş et al. 2019a, b).
Nile tilapia exhibited a differential MSTN gene expression during fasting and refeeding periods in this study. It was found that the chosen fasting periods during this study provoked MSTN expression levels, while refeeding reversed these findings.
Many studies have been conducted to investigate the actions of myostatin in skeletal muscle development and growth, and it has been discovered that myostatin inhibits total protein synthesis in C2C12 muscle cells in vitro (Taylor et al. 2001), while in vivo MSTN inactivation enhanced myofibrillar synthesis (Welle et al. 2006). Several studies stated that MSTN acts as a negative regulator of skeletal muscle development and growth in mammals and fish (Acosta et al. 2005; De Santis et al. 2012). Moreover, many models have suggested that the increment of endogenous myostatin expression is a crucial regulator of muscle atrophy (Carlson et al. 1999; Dasarathy et al. 2007; Chen et al. 2009; Plant et al. 2010). Moreover, Nebo et al. (2013) found that tilapia fingerlings (0.6 g) fasted for 5 days showed enhanced myostatin expression compared to the control fed group.
There is not much information concerning myogenin behavior under feed restriction in Nile tilapia. Our findings could help us understand the regulation of muscle growth catabolic conditions induced by fasting. Moreover, in the current study, MYOG expression increased in fasting fish, while it declined by refeeding. This means that myostatin activation has also been incorporated with the enhanced MYOG expression by fasting compared to the control fed group. Nebo et al. (2013) reported that warm water species such as the Nile tilapia revealed an elevation in MyoD mRNA levels during short periods of fasting (5 and 10 days) followed by refeeding. Studies have shown that myostatin regulates the differentiation process by inhibiting the action of myogenin, so MRF is most likely a significant target of endogenous myostatin (Joulia et al. 2003). However, such correlation between myostatin and myogenin expressions was not detected in the current study; Johansen and Overturf (2006) found that in rainbow trout (O. mykiss), both the myogenin and myostatin mRNA levels decreased after 30 days of fasting while increased after 14 days of refeeding, implying that myostatin may not control the expression of myogenin. Essentially, the mechanistic role of myostatin in regulating muscle growth in fish is not yet well understood. Research stated that myostatin regulatory mechanisms of muscle growth rely on the species of fish, stage of development, type of muscle, and state of nutrition (Roberts and Goetz 2001; Østbye et al. 2001; Patruno et al. 2008).
In the present trial, fasting also promoted enhancement of liver GH and NPYa expression levels but reduced liver IGF1 expression; however, refeeding progressively reversed this situation. These findings indicated that fasting periods restricted IGF1 production. Lavajoo et al. (2020) supported our conclusion by reporting that feed restriction depressed the growth promotion-involved components (GHR1, IGF1, IGFRb, IGFp5), while refeeding progressively reverted the situation to activate muscle recovery. Different starvation periods induced strong disruption of the GH/IGF axis by enhancing the expression levels of GH while reducing the expression of IGF-I as previously mentioned in trout (Sumpter et al. 1991; Gentil et al. 1996, Shimizu et al. 1999). Such a decline in IGF1 during the fasting period can inhibit muscle proliferation. It is often considered that the declined IGF-I enhances the increment of the GH levels. Likewise, when hepatic production of IGF-I was abolished in the mouse, the concentration of plasma IGF-I amounted to only 25% of the normal value, and these mice showed a threefold higher GH level (Sjogren et al. 1999). However, the lower expression of IGF-1 in fasted tilapia juveniles played a role in the elevated expression of MuRF1 and atrogin-1, therefore inducing muscle atrophy over a prolonged time and somatic growth block (Nebo et al. 2017). Also, Peterson and Waldbieser (2009) reported that fasted channel catfsh (Ictalurus punctatus) for 30 days showed weight reduction by about 60% with declined IGF1 mRNA in muscle. Interestingly, prolonged fasting periods markedly inhibited IGF-1 mRNA in the liver and muscle of numerous fish species (Gabillard et al. 2006; Pedroso et al. 2006; Peterson and Waldbieser 2009; Fuentes et al. 2003; Chen et al. 2019). It has been stated that fasting promotes the growth-inhibiting actions of GH rather than the growth-promoting actions (Norbeck et al. 2007) as GH levels are being shown to become dissociated under certain conditions such as malnutrition. Still, the correlation between IGF-I and growth persisted (Duan et al. 2010).
It has been described that refeeding stimulates the proliferation of fish myogenic cells (Rescan et al. 2007; Montserrat et al. 2007a, b), although many studies detected a positive correlation between circulating IGF-I and growth rates (Fox et al. 2006; De Santis and Jerry 2007). IGF1 plays a crucial role in vertebrates’ growth regulation (Sjögren et al. 1999; Chauvigné et al. 2003; Peterson and Waldbieser 2009; Vélez et al. 2017). It is well known in vertebrates that IGF-1 enhances PI3K/Akt cascade activation, thus enhancing protein synthesis and hypertrophic muscle growth (Engert et al. 1996; Glass 2003; Sacheck et al. 2004; Kandarian and Jackman 2006; Zhang et al. 2008; Duan et al. 2010; Fuentes et al. 2003; Rescan et al. 2017).
In this work, we also detected that starvation enhanced hepatic NPYa expression while refeeding normalizes its levels as compared to the control fed group. It was found that feed restriction induced NPYa mRNA expression levels in goldfish brain (Narnaware and Peter 2001), coho salmon (Oncorhynchus kisutch) (Silverstein et al. 1998) and Chinook salmon (Oncorhynchus tshawytscha), winter skate (Leucoraja ocellata) (MacDonald and Volkoff 2009), and Megalobrama amblycephala (Ji et al. 2015). Concomitantly, refeeding normalized NPY mRNA abundance following food deprivation in goldfish (Narnaware and Peter 2001) and in Megalobrama amblycephala (Ji et al. 2015), pointing to its appetite stimulatory effect on enhancing food intake in fish. Several hypotheses have been proposed to explain the increased growth rates following a period of fasting, such as an increase in feed intake (hyperphagia) (Hayward et al. 2000), protein synthesis (Bower et al. 2009), and hormonal responses (Gaylord and Gatlin 2001).
Among fasting refeeding regimes, group A (S1F5) recovered weight as the control group through restoring expression levels of IGF1, therefore achieving growth compensation. The results of our study confirm other reported observations in different fish species subjected to different fasting protocols as tilapia juveniles Oreochromis mossambicus (Fox et al. 2006), rainbow trout (Oncorhynchus mykiss) fry (Montserrat et al. 2007a, b), and rainbow trout adults subjected to 10 weeks period of fasting and 4–34 days of refeeding (Chauvigné et al. 2003). Similar results were recorded in hybrid tilapia (Oreochromis mossambicus × O. niloticus) and barramundi (Lates calcalifer) (Tian and Qin 2003).
Moreover, subsequent refeeding following the more extended fasting periods failed to recover weight and did not achieve growth compensation. Suppose the experiment involved longer refeeding periods after the long starvation periods. It may facilitate better fish growth and weight gain similar to that of the control. Interestingly, the marked increment of SGR in the second week of refeeding following the different fasting periods suggests that food deprivation following refeeding may be an effective strategy to induce compensatory growth. Still, it was effective for a short period (1 week) with longer refeeding. Still, more extended feed restriction periods require more prolonged refeeding periods to achieve complete growth compensation in Nile tilapia (O. niloticus).
Conclusion
In conclusion, this study revealed that fasted tilapia exhibited bodyweight reduction correlated with elevated expressions of some genes and dropped expressions of others, thus showing the inhibitory influence on muscle growth. However, subsequent refeeding may induce growth compensation, but the response depends on the length of starvation and refeeding periods and differential expression of muscle growth-related genes. The obtained results may help develop feeding strategies that jointly consider fish biology, appetite, and growth regulation to overcome short-term water quality problems that can benefit from reduced feeding as accelerated weight loss recovery may exist through growth compensation once refeeding is established in commercially cultured Nile tilapia (O. niloticus).
References
Abdo SE, Gewaily MS, Abo-Al-Ela HG, Almeer R, Soliman AA, Elkomy AH, Dawood MAO (2021) Vitamin C rescues inflammation, immunosuppression, and histopathological alterations induced by chlorpyrifos in Nile tilapia. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-11021-12711-11355
Abo-Raya MH, Alshehri KM, Abdelhameed RFA, Elbialy ZI, Elhady SS, Mohamed RA (2021) Assessment of growth-related parameters and immune-biochemical profile of Nile tilapia (oreochromis niloticus) fed dietary ulva fasciata extract. Aquac Res 00:1–14. https://doi.org/10.1111/are.15169
Acosta J, Carpio Y, Borroto I, González O, Estrada MP (2005) Myostatin gene silenced by RNAi show a zebrafish giant phenotype. J Biotechnol 119:324–331
Ali M, Nicieza A, Wootton RJ (2003) Compensatory growth in fishes: a response to growth depression. Fish Fish 4:147–190
Assan D, Mustapha UF, Chen H, Li Z, Peng Y, Li G (2021) The roles of neuropeptide Y (Npy) and peptide YY (Pyy) in teleost food intake: a mini review. Life 11:547. https://doi.org/10.3390/life11060547
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. Elsevier Health Sciences, Amsterdam
Barcellos LJG, Marqueze A, Trapp M, Quevedo RM, Ferreira D (2010) The effects of fasting on cortisol, blood glucose and liver and muscle glycogen in adult jundiá Rhamdia quelen. Aquaculture 300(1–4):231–236
Bower NI, Taylor RG, Johsnton IA (2009) Phasing of muscle gene expression with fasting-induced recovery growth in Atlantic salmon. Front Zool 6:18
Butler AA, Le Roith D (2001) Control of growth by the somatropic axis: growth hormone and the insulin like growth factors have related and independent roles. Ann Rev Physiol 63:141–164
Canosa LF, Chang JP, Peter RE (2007) Neuroendocrine control of growth hormone in fish. Gen Comp Endocrin 151:1–26
Carlson CJ, Booth FW, Gordon SE (1999) Skeletal muscle myostatin mRNA expression is fiber-type specific and increases during hindlimb unloading. Am J Physiol Regul Integr Comp Physiol 277:R601–R606
Chauvigné F, Gabillard JC, Weil C, Rescan PY (2003) Effect of refeeding on IGFI, IGFII, IGF receptors, FGF2, FGF6, and myostatin mRNA expression in rainbow trout myotomal muscle. Gen Comp Endocrinol 132:209–215
Chen Y, Cao L, Ye J, Zhu D (2009) Upregulation of myostatin gene expression in streptozotocin-induced type 1 diabetes mice is attenuated by insulin. Biochem Biophys Res Commun 388:112–116
Chen X, Wang G, Lu X, Xu P, Zeng S, Chen Z et al (2019) Molecular characterization and expression profiles of two insulin-like growth factor 1 receptors during fasting and refeeding in Siniperca chuatsi. Fish Sci 85:349–360. https://doi.org/10.1007/s12562-019-01290-7
Cho SH (2005) Compensatory growth of juvenile flounder Paralichthys olivaceus L. and changes in biochemical composition and body condition indices during starvation and after refeeding in winter season. J World Aquacult Soc 36(4):508–514
Colakoglu F, Donmez HH (2012) Effects of aflatoxin on liver and protective effectiveness of esterified glucomannan in merino rams. Sci World J 1–5. https://doi.org/10.1100/2012/462925
Costa LS, Rosa PV, Fortes-Silva R, Sánchez-Vázquez FJ, López –Olmeda JF (2015) Daily rhythms of the expression of genes from the somatotropic axis: the influence on tilapia (Oreochromisniloticus) of feeding and growth hormone administration at different times. Comp Biochem Physiol C. https://doi.org/10.1016/j.cbpc.2015.12.008
Dasarathy S, Muc S, Hisamuddin K, Edmison JM, Dodig M, McCullough AJ, Kalhan SC (2007) Altered expression of genes regulating skeletal muscle mass in the portacaval anastomosis rat. Am J Physiol Gastrointest Liver Physiol 292:G1105–G1113
Davis KB, Gaylord TG (2011) Effect of fasting on body composition and responses to stress in sunshine bass. Comp Biochem Physiol 158:30–36
De Santis C, Gomes GB, Jerry DR (2012) Abundance of myostatin gene transcripts and their correlation with muscle hypertrophy during the development of barramundi. Lates Calcarifer Comp Biochem Physiol B 163:101–107
De Santis C, Jerry DR (2007) Candidate growth genes in finfish - where should we be looking? Aquaculture 272(1–4):22–38
Duan C, Ren H, Gao S (2010) Insulin-like growth factors (IGFs), IGF receptors and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen Comp Physiol 167:344–351
Elkatatny NA, Elbialy ZI, El-Nahas AF, Mahmoud S (2016) Characterization of Myostatin gene in Nile tilapia (Oreochromisniloticus), the possible association of BsmI-exon 2 polymorphism with its growth. Am J Life Sci 4(3):82–86
Engert C, Berglund EB, Rosenthal N (1996) Proliferation precedes differentiation in IGF-I stimulated myogenesis. J Cell Biol 135:431–440
Fauconneau B, Alami-Durante H, Laroche M, Marcel J, Vallot D (1995) Growth and meat quality relations in carp. Aquaculture 129:265–297
Favero GC, Gimbo RY, Franco Montoya LN, Zanuzzo FS, Urbinati EC (2018) Fasting and refeeding lead to more efficient growth in lean pacu (Piaractus mesopotamicus). Aquac Res 49(1):359–366
Fox BK, Breves JP, Hirano T, Grau EG (2009) Effects of shortand long-term fasting on plasma and stomach ghrelin, and the growth hormone/insulin-like growth factor I axis in the tilapia, Oreochromis Mossambicus. Domest Anim Endocrinol 37:1–11
Fox BK, Riley LG, Hirano T, Grau EG (2006) Effects of fasting on growth hormone, growth hormone receptor, and insulin-like growth factor-I axis in seawater acclimated tilapia, Oreochromis Mossambicus. Gen Comp Endocrinol 148:340–347
Fuentes EN, Valdes JA, Molina A, Bjornsson BT (2003) Regulation of skeletal muscle growth in fish by the growth hormone- insulin-like growth factor system. Gen Comp Endocrinol 192:136–148
Gabillard J-C, Kamangar BB, Montserrat N (2006) Coordinated regulation of the GH/IGF system genes during refeeding in rainbow trout (Oncorhynchus mykiss). J Endocrinol 191:15–24
Gallardo-Collí A, Pérez-Fuentes M, Pérez-Rostro CI, Hernández-Vergara MP (2020) Compensatory growth of Nile tilapia Oreochromis niloticus, L. subjected to cyclic periods of feed restriction and feeding in a biofloc system. Aquac Res 51(5):1813–1823
Gao Y, Lee J-Y (2012) Compensatory responses of Nile tilapia Oreochromis niloticus under different feed-deprivation regimes. Fish Aquat Sci 15(4):305–311
Gao Y, Wang Z, Hur J-W, Lee J-Y (2015) Body composition and compensatory growth in Nile tilapia Oreochromis niloticus under different feeding intervals. Chin J Oceanol Limnol 33(4):945–956
Gaylord TG, Gatlin DM III (2001) Dietary protein and energy modifications to maximize compensatory growth of channel catfish (Ictalurus punctatus). Aquaculture 194:337–348
Gentil V, Martin P, Smal J, Le Bail P-Y (1996) Production of recombinant insulin-like growth factor-II in the development of a radioimmunoassay in rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 104:156–167
Glass DJ (2003) Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol 5(87–90):2003
Green BS, McCormick MI (1999) Infuence of larval feeding history on the body condition of Amphiprion melanops. J Fish Biol 55:1273–1289. https://doi.org/10.1111/j.1095-8649.1999.tb02075.x
Hagen Ø, Fernandes JMO, Solberg C, Johnston IA (2009) Expression of growth related genes in muscle during fasting and refeeding of juvenile Atlantic halibut Hippoglossus hippoglossus, L. Comp Biochem Physiol B 152:47–53
Hayward RS, Wang N, Noltie DB (2000) Group holding impedes compensatory growth of hybrid sunfish. Aquaculture 183:299–305
Hornick JL, Eenaeme CV, Gérard O, Dufrasne I, Istasse L (2000) Mechanisms of reduced and compensatory growth. Domest Anim Endocrinol 19:121–132
Hsieh S-L, Shiau S-Y (2000) Effects of diets containing different carbohydrates on starved condition in juvenile tilapia Oreochromis niloticus× O. aureus. Fish Sci 66(1):32–37
Ji W, Ping H-C, Wei K-J, Zhang G-R, Shi Z-C, Yang R-B, Zou G-W, Wang W-M (2015) Ghrelin, neuropeptide Y (NPY) and cholecystokinin (CCK) in blunt snout bream (Megalobrama amblycephala): cDNA cloning, tissue distribution and mRNA expression changes responding to fasting and refeeding. Gen Comp Endocrinol 2015(223):108–119
Jobling M (1995) Fish bioenergetics. Oceanogr Lit Rev 9(42):785
Jobling M (2010) Fish culture: feeds and feeding. Finfish aquaculture diversification, pp 61–87
Johansen KA, Overturf K (2006) Alterations in expression of genes associated with muscle metabolism and growth during nutritional restriction and refeeding and refeeding in rainbow trout. Comp Biochem Physiol B 144:119–127
Joulia D, Bernardi H, Garandel V, Rabenoelina F, Vernus B, Cabello G (2003) Mechanism involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp Cell Res 286:263–275
Kandarian SC, Jackman RW (2006) Intracellular signaling during skeletal muscle atrophy. Muscle Nerve 33:155–165
Kang JD, Kim S, Zhu HY, Jin L, Guo Q, Li XC (2017) Generation of cloned adult muscular pigs with myostatin gene mutation by genetic engineering. RSC Adv 7:12541–12549
Karataş T, Yildirim S, Arslan H (2019b) Effects of different concentrations of diazinon on 8-hydroxy-2-deoxyguanosine and histopathology, antioxidant enzyme, acetylcholinesterase activity and plasma metabolites in rainbow trout (Oncorhynchus mykiss). Int J Agric Biol 21(3):583–589. https://doi.org/10.17957/ijab/15.0932
Karataş T, Yildirim S, Arslan H, Aggul AG (2019b) The efects on brown trout (Salmo trutta fario) of different concentrations of deltamethrin. Comp BiochemPhysiol Part C 226:1–6. https://doi.org/10.1016/j.cbpc.2019.108606
Karatas T, Onalan S, Yildirim S (2021) Effects of prolonged fasting on levels of metabolites, oxidative stress, immune-related gene expression, histopathology, and DNA damage in the liver and muscle tissues of rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 47:1119–1132. https://doi.org/10.1007/s10695-021-00949-2
Lavajoo F, Perelló-Amorós M, Vélez EJ, Sánchez-Moya A, Balbuena-Pecino, Riera-Heredia N, Fernández-Borràs J, Blasco J, Navarro I, Capilla E, Gutiérrez J (2020) Regulatory mechanisms involved in muscle and bone remodeling during refeeding in gilthead sea bream. Sci Rep 10:184. https://doi.org/10.1038/s41598-019-57013-6
Liu W, Lu X, Jiang M, Wu F, Tian J, Yang C, . . . Wen H (2019) Effects of dietary manipulation on compensatory growth of juvenile genetically improved farmed tilapia (Oreochromis niloticus). Fish Physiol Biochem 45(1):21-32
Liu X, Zeng S, Liu S, Wang G, Lai H, Zhao X, Li G (2020) Identifying the related genes of muscle growth and exploring the functions by compensatory growth in Mandarin fish (Siniperca chuatsi). Front Physiol 11. https://doi.org/10.3389/fphys.2020.553563
Lv Q, Yuan L, Deng J, Chen M, Wang Y, Zeng J (2016) Efficient generation of myostatin gene mutated rabbit by CRISPR/Cas9. Sci Rep 6:25029
MacDonald E, Volkoff H (2009) Neuropeptide Y (NPY), cocaine- and amphetamine-regulated transcript (CART) and cholecystokinin (CCK) in winter skate (Raja Ocellata): CDNA cloning, tissue distribution and MRNA expression responses to fasting. Gen Comp Endocrinol 161:252–261. https://doi.org/10.1016/j.ygcen.2009.01.021
Martínez M, Guderley H, Dutil JD, Winger P, He P, Walsh SJ (2002) Condition, prolonged swimming performance and muscle metabolic capacities of cod Gadus morhua. J Exp Biol 206:503–511
Montserrat N et al (2007a) Distinct role of insulin and IGF-I and its receptors in white skeletal muscle during the compensatory growth of gilthead sea bream (Sparus aurata). Aquaculture 267:188–198
Montserrat N, Gabillard JC, Capilla E, Navarro MI, Gutierrez J (2007b) Role of Insulin, insulinlike growth factors, and muscle regulatory factors in the compensatory growth of the trout (Oncorhynchus mykiss). Gen Comp Endocrinol 150:462–427
Najafi A, Salati AP, Yavari V, Asadi F (2015) Effects of short term fasting and refeeding on some hematological and immune parameters in Mesopotamichthys sharpeyi (Günther, 1874) fingerlings. Iran J Sci Technol 39(A3):383–389
Narnaware YK, Peter RE (2001) Effects of food deprivation and refeeding on neuropeptide Y (NPY) MRNA levels in goldfish. Comp Biochem Physiol B Biochem Mol Biol 129:633–637. https://doi.org/10.1016/S1096-4959(01)00359-1
Nebo C, Gimbo RY, Kojima JT, Overturf K, Dal-Pai-Silva M, Portella MC (2018) Depletion of stored nutrients during fasting in Nile tilapia (Oreochromis niloticus) juveniles. J Appl Aquac 30(2):157–173
Nebo C, Portella MC, Carani FR, Almeida FLA, Padovani CR, Carvalho RF, Dal-Pai-Silva M (2013) Short periods of fasting followed by refeeding change the expression of muscle growth-related genes in juvenile Nile tilapia (Oreochromis niloticus). Comp Biochem Physiol - B Biochem Mol Biol 164:268–274. https://doi.org/10.1016/j.cbpb.2013.02.003
Nebo C, Overturf K, Brezas A, Dal-Pai-Silva M, Portella MC (2017) Alteration in expression of atrogenes and IGF-1 induced by fasting in Nile tilapia Oreochromis niloticus juveniles. Int Aquat Res 9:361–372. https://doi.org/10.1007/s40071-017-0182-1
Norbeck LA, Kittilson JD, Sheridan MA (2007) Resolving the growth promoting and metabolic effects of growth hormone: differential regulation of GH–IGF-I system components. Gen Comp Endocrinol 151:332–341. https://doi.org/10.1016/j.ygcen.2007.01.039
NRC (2011) National Research Council. Nutrient requirements of fish and shrimp. National Academies Press, Washington, D.C
Østbye TK, Galloway TF, Nielsen C, Gabestad I, Bardal T, Andersen Ø (2001) The two myostatin genes of Atlantic salmon (Salmo salar) are expressed in a variety of tissues. Eur J Biochem 268:5249–5257
Patruno M, Sivieri S, Poltronieri C, Sacchetto R, Maccatrozzo L, Martinello T, Funkenstein B, Radaelli G (2008) Real-time polymerase chain reaction, in situ hybridization and immunohistochemical localization of insulin-like growth factor-I and myostatin during development of Dicentrarchus labrax (Pisces: Osteichthyes). Cell Tissue Res 331:643–658
Pedroso FL, Jesus-Ayson EGT, Cortado HH, Hyodo S, Ayson FG (2006) Changes in mRNA expression of grouper (Epinephelus coioides) growth hormone and insulin-like growth factor I in response to nutritional status. Gen Comp Endocrinol 145:237–246. https://doi.org/10.1016/j.ygcen.2005.09.001
Peterson BC, Waldbieser GC (2009) Effects of fasting on IGF-I, IGF-II, and IGF-binding protein mRNA concentrations in channel catfsh (Ictalurus punctatus). Domest Anim Endocrinol 37:74–83
Plant PJ, Brooks D, Faughnan M, Bayley T, Bain J, Singer L, Correa J, Pearce D, Binnie M, Batt J (2010) Cellular markers of muscle atrophy in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 42(2010):461–471
Qiang J, He J, Yang H, Wang H, Kpundeh MD, Xu P, Zhu ZX (2014) Temperature modulates hepatic carbohydrate metabolic enzyme activity and gene expression in juvenile GIFT tilapia (Oreochromisniloticus) fed a carbohydrate-enriched diet. J Therm Biol 40:25–31
Reinecke M, Bjornsson BT, Dickhoff WW, McCormick SD, Navarro I, Power DM, Gutierrez J (2005) Growth hormone and insulin-like growth factors in fish: where we are and where to go. Gen Comp Endocrinol 142:20–24
Rescan PY et al (2007) Dynamic gene expression in fish muscle during recovery growth induced by a fasting-refeeding schedule. BMC Genomics 8:1–18
Rescan P-Y, Le Cam A, Ralliere C, Montfort J (2017) Global gene expression in muscle from fasted/refed trout reveals up-regulation of genes promoting myofibre hypertrophy but not myofibre production. BMC Genom Bio Med Cent 18(1):1–12. https://doi.org/10.1186/s12864-017-3837-9
Roberts SB, Goetz FW (2001) Differential skeletal muscle expression of myostatin across teleost species, and the isolation of multiple myostatin isoforms. FEBS Lett 491:212–216
Rønnestad I, Gomes AS, Murashita K, Angotzi R, Jönsson E, Volkoff H (2017) Appetite-controlling endocrine systems in teleosts. Front Endocrinol 8:73. https://doi.org/10.3389/fendo.00073
Sacheck JM, Ohtsuka A, Mclary SC, Goldberg AL (2004) IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab 287:591–601
Sadoul B, Vijayan MM (2016) Stress and growth. In: Fish physiology. Elsevier, pp 167–205. https://doi.org/10.1016/B978-0-12-802728-8.00005-9
Sakyi ME, Cai J, Ampofo-Yeboah A, Anokyewaa MA, Wang Z, Jian J (2021) Starvation and refeeding influence the growth, immune response, and intestinal microbiota of Nile tilapia (Oreochromis niloticus; Linnaeus 1758). Aquaculture 543:736959
Sänger AM, Stoiber W (2001) Muscle fiber diversity and plasticity. Fish Physiol 24:187–250
Shimizu M, Swanson P, Dickhoff WW (1999) Free and protein-bound insulinlikegrowthfactor-I (IGF-I)and IGF-binding proteins in plasma of coho salmon, Oncorhynchus Kisutch. Gen Comp Endocrinol 115:398–405
Silverstein JT, Breininger J, Baskin DG, Plisetskaya EM (1998) Neuropeptide Y-like gene expression in the salmon brain increases with fasting. Gen Comp Endocrinol 220:78–87. https://doi.org/10.1006/gcen.1998.7058
Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, Leroith D, Tornell J, Isaksson OGP, Jansson JO et al (1999) Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. PNAS 96:7088–7092
Sjögren K et al (1999) Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci USA 96:7088–7092
Soengas JL, Cerdá-Reverter JM, Delgado MJ (2018) Central regulation of food intake in fish: an evolutionary perspective. J Mol Endocrinol 60:R171–R199. https://doi.org/10.1530/JME-17-0320
Sridee N, Boonanuntanasarn S (2012) The effects of food deprivation on hematological indices and blood indicators of liver function in Oxyleotris marmorata. Int J Med Biol Sci 6(5):254–258
Storch V, Juario JV (1983) The efect of starvation and subsequent feeding on the hepatocytes of Chanos chanos (Forsskal) fingerlings and fry. J Fish Biol 23:95–103. https://doi.org/10.1111/j.1095-8649.1983.tb02884.x
Sumpter JP, Le Bail P-Y, Pickering AD, Pottinger TG, Carragher JF (1991) The effect of starvation on growth and plasma growth hormone concentrations of rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol 83:94–102
Taylor WE, Bhasin S, Artaza J, Byhower F, Azam M, Willard DH Jr, Kull FC Jr, Gonzalez-Cadavid N (2001) Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells. Am J Physiol Endocrinol Metab 2001(280):E221–E228
Tian X, Qin JG (2003) A single phase of food deprivation provoked compensatory growth in barramundi Lates calcarifer. Aquaculture 224:169–179
Torfi Mozanzadeh M, Marammazi J, Yaghoubi M, Yavari V, Agh N, Gisbert E (2017) Somatic and physiological responses to cyclic fasting and refeeding periods in sobaity sea bream (S paridentex hasta, V alenciennes 1830). Aquac Nutr 23(1):181–191
Vélez EJ et al (2017) Understanding fish muscle growth regulation to optimize aquaculture production. Aquaculture 467:28–40
Volkoff H (2016) The neuroendocrine regulation of food intake in fish: a review of current knowledge. Front Neurosci 10:540. https://doi.org/10.3389/fnins.2016.00540
Volkoff H, Canosa LF, Unniappan S, Cerdá-Reverter JM, Bernier NJ, Wang YK, Cui Y, Yang YX, Cai FS (2000) Compensatory growth in hybrid tilapia, Oreochromis mossambicus and O. niloticus, reared in seawater. Aquaculture 189:101–108
Wang Y, Li C, Qin JG, Han H (2009) Cyclical feed deprivation and refeeding fails to enhance compensatory growth in Nile tilapia, Oreochromis Niloticus L. Aquac Res 40(2):204–210
Wasielesky W, Froes C, Fóes G, Krummenauer D, Lara G, Poersch L (2013) Nursery of Litopenaeus vannamei reared in a biofloc system: the effect of stocking densities and compensatory growth. J Shellfish Res 32(3):799–806
Weatherley AH, Gill HS (1985) Dynamics of increase in muscle fibres in fishes in relation to size and growth. Experientia 41:353–354
Welle S, Bhatt K, Pinkert CA (2006) Myofibrillar protein synthesis in myostatin-deficient mice. Am J Physiol Endocrinol Metab 290:E409–E415
Won ET, Borski RJ (2013) Endocrine regulation of compensatory growth in fish. Front Endocrinol 4
Yan P, Jia J, Yang G, Wang D, Sun C, Li W (2017) Duplication of neuropeptide Y and peptide YY in Nile tilapia Oreochromis niloticus and their roles in food intake regulation. Peptides 88:97–105
Yang Y, Zhou H, Shu H, Zhong D, Zhang M, Xia JH (2019) Effect of fasting and subsequent refeeding on the transcriptional profiles of brain in juvenile Spinibarbus hollandi. PLoS ONE 14(3):e0214589. https://doi.org/10.1371/journal.pone.0214589
Zhang G, Swank DM, Rome LC (1996) Quantitative distribution of muscle fiber types in the scup Stenotomus chrysops. J Morphol 229:71–81
Zhang XD, Zhu YF, Cai LS, Wu TX (2008) Effects of fasting on the meat quality and antioxidant defenses of market-size farmed large yellow croaker (Pseudosciaena crocea). Aquaculture 280:136–139
Zhao X, Li M, Xu S, Sun J, Liu G (2019) Expression of Myostatin (Mstn) and Myogenin (Myog) Genes in Zi And Rhine Goose and Their Correlation with Carcass Traits. Braz J Poult Sci 21(1). https://doi.org/10.1590/1806-9061-2017-0710
Zheng Y, Cheng X, Tang H (2015) Effects of starvation and refeeding on digestive enzyme activity of Megalobrama pellegrini. Adv J Food Sci Technol 7(4):230–234
Acknowledgements
The authors would like to acknowledge the Biotechnology Lab., Faculty of Aquatic and Fisheries Sciences, Kafrelsheikh University, where the molecular analysis was performed.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Z.E. is responsible for conceptualization, molecular analysis, review and editing the final version, and publishing paper. S.G. performed the experiment, sampling, and laboratory investigation. I.I.A. is responsible for supervision and validation of the study. M.S. is responsible for resources and software analysis of data. A.S.S. is responsible for data curation and statistical analysis. A.A.A. is responsible for study visualization and supervision of the whole experiment. D.H.A. is responsible for methodology, including pathological examination and validation of the study, and was a significant contributor to writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All the experimental procedures are performed according to the Egyptian ethical codes for studies on experimental animals and approved by the Animal Ethical Committee, Kafrelsheikh University (Number: IAACUC-KSU-2020–22).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elbialy, Z.I., Gamal, S., Al-Hawary, I.I. et al. Exploring the impacts of different fasting and refeeding regimes on Nile tilapia (Oreochromis niloticus L.): growth performance, histopathological study, and expression levels of some muscle growth-related genes. Fish Physiol Biochem 48, 973–989 (2022). https://doi.org/10.1007/s10695-022-01094-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-022-01094-0