Abstract
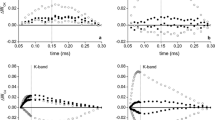
In the present study, we analysed the photosynthetic performance in five varieties of Vigna radiata, viz. vars RMG 268, K-851, RMG 492, RMG 975 and Anand using chlorophyll fluorescence parameters. We observed that var. RMG 268 tended to reach highest effective quantum yield of PSII [∆F/Fm′], maximum apparent electron transport rate [ETRmax] and saturating photosynthetically active photon flux density [PPFDsat], followed by var. K-851. Thus as judged by its photosynthetic performance, ecophysiologically var. RMG 268 seems to be better adapted to the semi-arid environment of the state of Rajasthan, India. On the contrary, var. Anand was least adapted to its environment as indicated by lowest ETRmax, PPFDsat and ΔF/Fm′ values. The activities of certain antioxidant enzymes of Vigna radiata in response to drought were also examined in var. RMG 268 and var. Anand. The increased activities of antioxidant enzymes, presumed to limit cellular damage, were observed in var. RMG 268. Cellular malondialdehyde content signal indicators of lipid peroxidation were much higher in var. Anand compared to var. RMG 268. These data revealed that var. RMG 268 had high resistance to environmental and drought conditions and thus substantiated our results obtained on the basis of plant performance.





Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Method Enzymol 105:121–126
Anjum NA, Umar S, Iqbal M, Khan NA (2008) Growth characteristics and antioxidant metabolism of moongbean genotypes differing in photosynthetic capacity subjected to water deficit stress. J Plant Interact 3:127–136
Ashraf M, Harris PJC (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51(2):163–190
Batra NG, Sharma V, Kumari N (2014) Drought-induced changes in chlorophyll fluorescence, photosynthetic pigments, and thylakoid membrane proteins of Vigna radiata. J Plant Interact 9(1):712–721
Batra NG, Kumari N, Sharma V (2016) Photosynthetic performance of Ocimum sanctum morphotypes in a semiarid region. J Herbs Spices Med Plants 22(3):211–224
De Vos CHR, Schat H, Vooijs R, Ernst WHO (1989) Copper- induced damage to the permeability barrier in roots of Silence cucubalus. J Plant Physiol 135:164–179
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 299:185–212
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Gonzalez A, Martın I, Ayerbe L (2008) Yield and osmotic adjustment capacity of barley under terminal water-stress conditions. J Agron Crop Sci 194:81–91
Grant RH, Slusser JR (2004) Estimation of photosynthetic photon flux density from 368-nm spectral irradiance. J Atmos Ocean Technol 21:481–487
Guo Z, Ou W, Lu S, Zhong Q (2006) Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol Biochem 44:828–836
Habibi D, Boojar MMA, Mahmoudi A, Ardakani MR, Taleghani DF (2004) Antioxidative enzymes in sunflower subjected to drought stress. In: Proceeding of the 4th international crop science congress, 26 Sept–10 Oct, Brisbane, Australia
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. In: Hoagland DR, Arnon DI (eds) Circular No. 347: California Agriculture Experiment Station. The College of Agriculture, University of California, Berkeley, pp 1–32
Hong PC, Jun ZS, Zhong GZ, Bao-Shan WW (2005) NaCl treatment markedly enhances H2O2-scavenging system in leaves of halophyte Suaeda salsa. Physiol Plant 125:490–499
Horton P, Ruban AV, Walters RG (1996) Regulation of light harvesting in green plants. Ann Rev Plant Physiol Plant Mol Biol 47:655–684
Hossain MA, Asada K (1984) Purification of dehydroascorbate reductase from spinach and its characterization as a thiol enzyme. Plant Cell Physiol 25:85–92
Johnson GN, Young AJ, Scholes JD, Horton P (1993) The dissipation of excess excitation energy in British plant species. Plant Cell Environ 16:673–679
Kumari N, Sharma V, Mikosch M, Unfried C, Gessler A, Fischer-Schliebs E, Luettge U (2005) Seasonal photosynthetic performance and nutrient relations of Butea monosperma TAUB. in comparison to two other woody species of a seasonal deciduous forest in S.E-Rajasthan and to planted trees in the area. Ind Jour Fores 26(2):116–126
Lambrides J, Godwin ID (2007) Mungbean. In: Kole C (ed) Pulses, sugar and tuber crops. Genome mapping and molecular breeding in plants. Springer, Heidelberg, pp 69–90
Manivannan P, Jaleel CA, Somasundaram R, Panneerselvam R (2008) Osmoregulation and antioxidant metabolism in drought-stressed Helianthus annuus under triadimefon drenching. C R Biol 331:418–425
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51(345):659–668
Mittal S, Kumari N, Sharma V (2011) Differential responses of seven contrasting species to high light using pigment and chlorophyll a fluorescence. J Stress Physiol Biochem 7(2):20–33
Mittal S, Kumari N, Sharma V (2012) Differential response of salt stress on Brassica juncea: photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiol Biochem 54:17–26
Nayyar H, Gupta D (2006) Differential sensitivity of C3 and C4 plants to water deficit stress: association with oxidative stress and antioxidants. Environ Exp Bot 58:106–113
Osmond CB (1994) What is photoinhibition? Some insights from comparisons of shade and sun plants. In: Baker NR, Bowyer JR (eds) Photoinhibition of photosynthesis, from molecular mechanism to the field. Bios Scientific Publishers, Oxford, pp 229–332
Putter J (1974) Peroxidases. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 685–690
Rascher U, Liebig M, Luettge U (2000) Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ 23:1397–1405
Sayed OH (2003) Chlorophyll fluorescence as a tool in cereal crop research. Photosynthetica 41:321–330
Schonfeld MA, Johnson RC, Carver BF, Mornhinweg DW (1988) Water relations in winter wheat as drought resistance indicators. Crop Sci 28:526–531
Schreiber U, Bilger W (1993) Progress in the chlorophyll fluorescence research: major developments during the last years in retrospect. Prog Bot 5:151–173
Tohidi-Moghaddam HR, Shirani-Rad AR, Noormohammadi G, Habibi D, Boojar MMA (2009) Effect of super absorbent application on antioxidant enzyme activities in canola [Brassica napus L.] cultivars under water stress conditions. Am J Agric Biol Sci 4:215–223
Wadhwa R, Kumari N, Sharma V (2010) Varying light regimes in naturally growing Jatropha curcus: pigment, proline and photosynthetic performance. J Stress Physiol Biochem 6(4):66–80
Wadhwa R, Kumari N, Sharma V (2013) Water deficit induced changes in Chlorophyll a fluorescence and proline content in Jatropha curcus. Res J Biotechnol 8(1):33–40
Wu N, Guan Y, Shi Y (2011) Effect of water stress on physiological traits and yield in rice backcross lines after anthesis. Energy Proc 5:255–260
Yadav SK, Tiwari YK, Kumar P, Jyothi Lakshmi DN, Vanaja M, Maheswari M (2016) Genotypic variation in physiological traits under high temperature stress in maize. Agric Res 5:119–126
Yang F, Miao LF (2010) Adaptive responses to progressive drought stress in two poplar species originating from different altitudes. Silva Fennica 44(1):23–37
Acknowledgements
The authors gratefully acknowledge the assistance of Krishi Vigyan Kendra, Banasthali University, for providing seeds for the experiments.
Author information
Authors and Affiliations
Contributions
NK and NGB have substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, VS had significant contribution in drafting the article and revising it critically for important intellectual content, and final approval of the version is to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no conflict of interest exists.
Informed Consent
For this type of study, formal consent is not required.
Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kumari, N., Batra, N.G. & Sharma, V. Photosynthetic Performance and Drought-Induced Changes in Activity of Antioxidative Enzymes in Different Varieties of Vigna radiata. Agric Res 7, 1–9 (2018). https://doi.org/10.1007/s40003-018-0291-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40003-018-0291-0