Abstract
BACKGROUND:
Intra-articular injection is a classic strategy for the treatment of early osteoarthritis (OA). However, the local delivery of traditional therapeutic agents has limited benefits for alleviating OA. Exosomes, an important type of extracellular nanovesicle, show great potential for suppressing cartilage destruction in OA to replace drugs and stem cell-based administration.
METHODS:
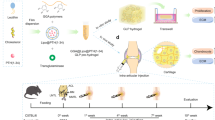
In this study, we developed a thermosensitive, injectable hydrogel by in situ crosslinking of Pluronic F-127 and hyaluronic acid, which can be used as a slow-release carrier to durably retain primary chondrocyte-derived exosomes at damaged cartilage sites to effectively magnify their reparative effect.
RESULTS:
It was found that the hydrogel can sustainedly release exosomes, positively regulate chondrocytes on the proliferation, migration and differentiation, as well as efficiently induce polarization of M1 to M2 macrophages. Intra-articular injection of this exosomes-incorporated hydrogel significantly prevented cartilage destruction by promoting cartilage matrix formation. This strategy also displayed a regenerative immune phenotype characterized by a higher infiltration of CD163+ regenerative M2 macrophages over CD86+ M1 macrophages in synovial and chondral tissue, with a concomitant reduction in pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) and increase in anti-inflammatory cytokine (IL-10) in synovial fluid.
CONCLUSION:
Our results demonstrated that local sustained-release primary chondrocyte-derived exosomes may relieve OA by promoting the phenotypic transformation of macrophages from M1 to M2, which suggesting a great potential for the application in OA.









Similar content being viewed by others
References
Peat G, Thomas MJ. Osteoarthritis year in review 2020: epidemiology & therapy. Osteoarthritis Cartilage. 2021;29:180–9.
Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338:917–21.
Bai H, Zhao Y, Wang C, Wang Z, Wang J, Liu H, et al. Enhanced osseointegration of three-dimensional supramolecular bioactive interface through osteoporotic microenvironment regulation. Theranostics. 2020;10:4779–94.
Zhao Y, Li Z, Jiang Y, Liu H, Feng Y, Wang Z, et al. Bioinspired mineral hydrogels as nanocomposite scaffolds for the promotion of osteogenic marker expression and the induction of bone regeneration in osteoporosis. Acta Biomater. 2020;113:614–26.
Yu W, Hu B, Boakye-Yiadom KO, Ho W, Chen Q, Xu X, et al. Injectable hydrogel mediated delivery of gene-engineered adipose-derived stem cells for enhanced osteoarthritis treatment. Biomaterials Science. 2021;9:7603–16.
Nguyen TH, Duong CM, Nguyen XH, Than UTT. Mesenchymal stem cell-derived extracellular vesicles for osteoarthritis treatment: extracellular matrix protection, chondrocyte and osteocyte physiology, pain and inflammation management. Cells. 2021;10:2887.
Sanghani-Kerai A, Black C, Cheng SO, Collins L, Schneider N, Blunn G, et al. Clinical outcomes following intra-articular injection of autologous adipose-derived mesenchymal stem cells for the treatment of osteoarthritis in dogs characterized by weight-bearing asymmetry. Bone Joint Res. 2021;10:650–8.
Chen CF, Hu CC, Wu CT, Wu HH, Chang CS, Hung YP, et al. Treatment of knee osteoarthritis with intra-articular injection of allogeneic adipose-derived stem cells (ADSCs) ELIXCYTE ®: a phase I/II, randomized, active-control, single-blind, multiple-center clinical trial. Stem Cell Res Ther. 2021;12:562.
Liu X, Yang Y, Li Y, Niu X, Zhao B, Wang Y, et al. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9:4430–8.
Chang YH, Liu HW, Wu KC, Ding DC. Mesenchymal stem cells and their clinical applications in osteoarthritis. Cell Transplant. 2016;25:937–50.
Ha CW, Park YB, Kim SH, Lee HJ. Intra-articular mesenchymal stem cells in osteoarthritis of the knee: a systematic review of clinical outcomes and evidence of cartilage repair. Arthroscopy. 2019;35:277–88.e2.
Savkovic V, Li H, Seon JK, Hacker M, Franz S, Simon JC. Mesenchymal stem cells in cartilage regeneration. Curr Stem Cell Res Ther. 2014;9:469–88.
Doyle EC, Wragg NM, Wilson SL. Intraarticular injection of bone marrow-derived mesenchymal stem cells enhances regeneration in knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2020;28:3827–42.
Lee Y, Park YS, Choi NY, Kim YI, Koh YG. Proteomic analysis reveals commonly secreted proteins of mesenchymal stem cells derived from bone marrow, adipose tissue, and synovial membrane to show potential for cartilage regeneration in knee osteoarthritis. Stem Cells Int. 2021;2021:6694299.
Zhang J, Rong Y, Luo C, Cui W. Bone marrow mesenchymal stem cell-derived exosomes prevent osteoarthritis by regulating synovial macrophage polarization. Aging (Albany NY). 2020;12:25138–52.
Mianehsaz E, Mirzaei HR, Mahjoubin-Tehran M, Rezaee A, Sahebnasagh R, Pourhanifeh MH, et al. Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res Ther. 2019;10:340.
Lee JC, Choe SY. Gene alterations after human adipose-derived stem cell-derived exosome injection in monosodium iodoacetate-induced osteoarthritis rats by microarray analysis. J Anat Soc India. 2020;69:37–47.
Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B, et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017;8:189.
Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7:180–95.
Zheng L, Wang Y, Qiu P, Xia C, Fang Y, Mei S, et al. Primary chondrocyte exosomes mediate osteoarthritis progression by regulating mitochondrion and immune reactivity. Nanomedicine (Lond). 2019;14:3193–212.
Nikhil A, Kumar A. Evaluating potential of tissue-engineered cryogels and chondrocyte derived exosomes in articular cartilage repair. Biotechnol Bioeng. 2022;119:605–25.
Patel JM, Loebel C, Saleh KS, Wise BC, Bonnevie ED, Miller LM, et al. Stabilization of damaged articular cartilage with hydrogel-mediated reinforcement and sealing. Adv Healthc Mater. 2021;10:e2100315.
Antich C, Jiménez G, de Vicente J, López-Ruiz E, Chocarro-Wrona C, Griñán-Lisón C, et al. Development of a biomimetic hydrogel based on predifferentiated mesenchymal stem-cell-derived ECM for cartilage tissue engineering. Adv Healthc Mater. 2021;10:e2001847.
Zhao Y, Li Z, Song S, Yang K, Liu H, Yang Z, et al. Skin-inspired antibacterial conductive hydrogels for epidermal sensors and diabetic foot wound dressings. Adv Funct Mater. 2019; 29:1901474.
Li Z, Zhao Y, Liu H, Ren M, Wang Z, Wang X, et al. pH-responsive hydrogel loaded with insulin as a bioactive dressing for enhancing diabetic wound healing. Mate Des. 2021; 210:110104.
Bai H, Kyu-Cheol N, Wang Z, Cui Y, Liu H, Liu H, et al. Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers. J Tissue Eng. 2020;11:2041731420947242.
Gosset M, Berenbaum F, Thirion S, Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 2008;3:1253–60.
Wang K, Dong R, Tang J, Li H, Dang J, Zhang Z, et al. Exosomes laden self-healing injectable hydrogel enhances diabetic wound healing via regulating macrophage polarization to accelerate angiogenesis. Chem Eng J. 2022; 430;132664.
Jung YS, Park W, Park H, Lee DK, Na K. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr Polym. 2017;156:403–8.
Hsieh HY, Lin WY, Lee AL, Li YC, Chen YJ, Chen KC, et al. Hyaluronic acid on the urokinase sustained release with a hydrogel system composed of poloxamer 407: HA/P407 hydrogel system for drug delivery. Plos One. 2020;15:e0227784.
Kang ML, Jeong SY, Im GI. Hyaluronic acid hydrogel functionalized with self-assembled micelles of amphiphilic PEGylated kartogenin for the treatment of osteoarthritis. Tissue Eng Part A. 2017;23:630–9.
Bai H, Cui Y, Wang C, Wang Z, Luo W, Liu Y, et al. 3D printed porous biomimetic composition sustained release zoledronate to promote osteointegration of osteoporotic defects. Mater Des. 2020;189:108513.
Yang HM, Yoon HY, Kim CH, Goo YT, Choi IJ, Park SG, et al. Poloxamer 407-based floating hydrogels for intravesical instillation: statistical optimization using central composite design, gel erosion, and drug release. Bull Korean Chem Soc. 2021;42:72–9.
Tundisi LL, Yang R, Borelli LPP, Alves T, Mehta M, Chaud MV, Mazzola PG, Kohane DS. Enhancement of the mechanical and drug-releasing properties of Poloxamer 407 hydrogels with casein. Pharm Res. 2021;38:515–22.
He Z, Wang B, Hu C, Zhao J. An overview of hydrogel-based intra-articular drug delivery for the treatment of osteoarthritis. Colloids Surf B Biointerfaces. 2017;154:33–9.
Henrotin Y, Raman R, Richette P, Bard H, Jerosch J, Conrozier T, et al. Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Semin Arthritis Rheum. 2015;45:140–9.
Yano F, Ohba S, Murahashi Y, Tanaka S, Saito T, Chung UI. Runx1 contributes to articular cartilage maintenance by enhancement of cartilage matrix production and suppression of hypertrophic differentiation. Sci Rep. 2019;9:7666.
Lefebvre V, Angelozzi M, Haseeb A. SOX9 in cartilage development and disease. Curr Opin Cell Biol. 2019;61:39–47.
Haseeb A, Kc R, Angelozzi M, de Charleroy C, Rux D, Tower RJ, et al. SOX9 keeps growth plates and articular cartilage healthy by inhibiting chondrocyte dedifferentiation/osteoblastic redifferentiation. Proc National Acad Sci U S A. 2021;118:e2019152118.
Xu X, Liang Y, Li X, Ouyang K, Wang M, Cao T, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539.
Lee GW, Thangavelu M, Choi MJ, Shin EY, Kim HS, Baek JS, et al. Exosome mediated transfer of miRNA-140 promotes enhanced chondrogenic differentiation of bone marrow stem cells for enhanced cartilage repair and regeneration. J Cell Biochem. 2020;121:3642–52.
Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27.
Su N, Hao Y, Wang F, Hou W, Chen H, Luo Y. Mesenchymal stromal exosome-functionalized scaffolds induce innate and adaptive immunomodulatory responses toward tissue repair. Sci Adv. 2021;7:eabf7207.
He Y, Tian M, Li X, Hou J, Chen S, Yang G, et al. A hierarchical-structured mineralized nanofiber scaffold with osteoimmunomodulatory and osteoinductive functions for enhanced alveolar bone regeneration. Adv Healthc Mater. 2022;11:e2102236.
Li X, Wang X, Liu Q, Yan J, Pan D, Wang L, et al. ROS-responsive boronate-stabilized polyphenol-poloxamer 188 assembled dexamethasone nanodrug for macrophage repolarization in osteoarthritis treatment. Adv Healthc Mater. 2021;10:e2100883.
Youn YJ, Shrestha S, Lee YB, Kim JK, Lee JH, Hur K, et al. Neutrophil-derived trail is a proinflammatory subtype of neutrophil-derived extracellular vesicles. Theranostics. 2021;11:2770–87.
Camarero-Espinosa S, Carlos-Oliveira M, Liu H, Mano JF, Bouvy N, Moroni L. 3D Printed dual-porosity scaffolds: the combined effect of stiffness and porosity in the modulation of macrophage polarization. Adv Healthc Mater. 2022;11:e2101415.
Bai JY, Li Y, Xue GH, Li KR, Zheng YF, Zhang ZQ, et al. Requirement of G alpha i1 and G alpha i3 in interleukin-4-induced signaling, macrophage M2 polarization and allergic asthma response. Theranostics. 2021;11:4894–909.
Mao G, Xu Y, Long D, Sun H, Li H, Xin R, et al. Exosome-transported circRNA_0001236 enhances chondrogenesis and suppress cartilage degradation via the miR-3677–3p/Sox9 axis. Stem Cell Res Ther. 2021;12:389.
Duan L, Xu X, Xu L, Chen H, Li X, Alahdal M, et al. Exosome-mediated drug delivery for cell-free therapy of osteoarthritis. Curr Med Chem. 2021;28:6458–83.
Atukorala I, Kwoh CK, Guermazi A, Roemer FW, Boudreau RM, Hannon MJ, et al. Synovitis in knee osteoarthritis: a precursor of disease? Ann Rheum Dis. 2016;75:390–5.
Kwon JY, Lee SH, Na HS, Jung K, Choi J, Cho KH, et al. Kartogenin inhibits pain behavior, chondrocyte inflammation, and attenuates osteoarthritis progression in mice through induction of IL-10. Sci Rep. 2018;8:13832.
Hu QC, Ecker M. Overview of MMP-13 as a promising target for the treatment of osteoarthritis. Int J Mol Sci. 2021;22:1742.
Lan QM, Lu RB, Chen HM, Pang YF, Xiong F, Shen C, et al. MMP-13 enzyme and pH responsive theranostic nanoplatform for osteoarthritis. J Nanobiotechnology. 2020;18:117.
Fahy N, de Vries-van Melle ML, Lehmann J, Wei W, Grotenhuis N, Farrell E, et al. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthritis Cartilage. 2014;22:1167–75.
Kwon JY, Lee SH, Na HS, Jung K, Choi J, Cho KH, et al. Kartogenin inhibits pain behavior, chondrocyte inflammation, and attenuates osteoarthritis progression in mice through induction of IL-10. Sci Rep. 2018;8:13832.
Ding J, Chen B, Lv T, Liu X, Fu X, Wang Q, et al. Bone marrow mesenchymal stem cell-based engineered cartilage ameliorates polyglycolic acid/polylactic acid scaffold-induced inflammation through M2 polarization of macrophages in a pig model. Stem Cells Transl Med. 2016;5:1079–89.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (82072533), the Science and Technology Committee of Fengxian District, Shanghai (20201501).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical statement
All animal procedures were performed in accordance with the guidelines for Care and Use of Laboratory Animal Experience and approved by the Animal Care and Use Ethics Committee of Northern Jiangsu People's Hospital (IACUC no. 20170312001).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sang, X., Zhao, X., Yan, L. et al. Thermosensitive Hydrogel Loaded with Primary Chondrocyte-Derived Exosomes Promotes Cartilage Repair by Regulating Macrophage Polarization in Osteoarthritis. Tissue Eng Regen Med 19, 629–642 (2022). https://doi.org/10.1007/s13770-022-00437-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-022-00437-5