Abstract
Background:
The delivery of recombinant human bone morphogenetic protein 2 (rhBMP2) by using various carriers has been used to successfully induce bone formation in many animal models. However, the effect of multiple administration of rhBMP2 on bone formation and BMP2 antibody production has not been determined. Our aim was to examine the bone formation activity of rhBMP2 and serum levels of anti-BMP2 antibodies following the repeated administration of rhBMP2 in mice.
Methods:
Absorbable collagen sponges or polyphosphazene hydrogels containing rhBMP2 were subcutaneously implanted or injected into one side on the back of six-week-old C57BL/6 mice. Three or 4 weeks later, the same amount of rhBMP2 was administered again with the same carrier into the subcutaneous regions on the other side of the back or into calvarial defects. The effects of a single administration of rhBMP2 on the osteoinductive ability in the ectopic model were compared with those of repeated administrations. In vivo ectopic or orthotopic bone formation was evaluated using microradiography and histological analyses. Serum concentrations of anti-rhBMP2 antibodies were measured by ELISAs.
Results:
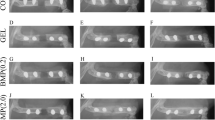
Re-administration of the same amount of rhBMP2 into the subcutaneous area showed a comparable production of ectopic bone as after the first administration. The bone forming ability of repeated rhBMP2 administrations was equal to that of single rhBMP2 administration. The administration of rhBMP2 into calvarial defects, following the first subcutaneous administration of rhBMP2 on the back, completely recovered the defect area with newly regenerated bone within 3 weeks. Repeated administration of rhBMP2 at 4-week intervals did not significantly alter the serum levels of anti-BMP2 antibodies and did not induce any inflammatory response. The serum obtained from rhBMP2-exposed mice had no effect on the ability of rhBMP2 to induce osteogenic gene expressions in MC3T3-E1.
Conclusion:
We suggest that the osteoinductive ability of rhBMP2 is not compromised by repeated administrations. Thus, rhBMP2 can be repeatedly used for bone regeneration at various sites within a short duration.




Similar content being viewed by others
References
Salazar VS, Gamer LW, Rosen V. BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol. 2016;12:203–21.
Carreira AC, Lojudice FH, Halcsik E, Navarro RD, Sogayar MC, Granjeiro JM. Bone morphogenetic proteins: facts, challenges, and future perspectives. J Dent Res. 2014;93:335–45.
Brown KV, Li B, Guda T, Perrien DS, Guelcher SA, Wenke JC. Improving bone formation in a rat femur segmental defect by controlling bone morphogenetic protein-2 release. Tissue Eng Part A. 2011;17:1735–46.
Rosen V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 2009;20:475–80.
Schmidt-Bleek K, Willie BM, Schwabe P, Seemann P, Duda GN. BMPs in bone regeneration: less is more effective, a paradigm-shift. Cytokine Growth Factor Rev. 2016;27:141–8.
Kim JK, Kim SE, Shim KM, Bae CS, Choi SH, Kang SS. Effects of mesenchymal stem cells treated with BMP-2 and VEGF on regeneration of large bone defects. J Biomed Res. 2014;15:24–31.
Kisiel M, Klar AS, Ventura M, Buijs J, Mafina MK, Cool SM, et al. Complexation and sequestration of BMP-2 from an ECM mimetic hyaluronan gel for improved bone formation. PLoS One. 2013;8:e78551.
Granjeiro JM, Oliveira RC, Bustos-Valenzuela JC, Sogayar MC, Taga R. Bone morphogenetic proteins: from structure to clinical use. Braz J Med Biol Res. 2005;38:1463–73.
James AW, LaChaud G, Chen J, Asatrian G, Nguyen V, Zhang X, et al. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng Part B Rev. 2016;22:284–97.
Neovius E, Lemberger M, Docherty Skogh AC, Hilborn J, Engstrand T. Alveolar bone healing accompanied by severe swelling in cleft children treated with bone morphogenetic protein-2 delivered by hydrogel. J Plast Reconstr Aesthet Surg. 2013;66:37–42.
Ritting AW, Weber EW, Lee MC. Exaggerated inflammatory response and bony resorption from BMP-2 use in a pediatric forearm nonunion. J Hand Surg Am. 2012;37:316–21.
Burkus JK, Gornet MF, Glassman SD, Slosar PJ, Rosner MK, Deckey JE, et al. Blood serum antibody analysis and long-term follow-up of patients treated with recombinant human bone morphogenetic protein-2 in the lumbar spine. Spine (Phila Pa 1976). 2011;36:2158–67.
Seo BB, Choi H, Koh JT, Song SC. Sustained BMP-2 delivery and injectable bone regeneration using thermosensitive polymeric nanoparticle hydrogel bearing dual interactions with BMP-2. J Control Release. 2015;209:67–76.
Lee SE, Ryu PY, Kim SY, Kim YR, Koh JT, Kim OJ, et al. Production of Vibrio vulnificus hemolysin in vivo and its pathogenic significance. Biochem Biophys Res Commun. 2004;324:86–91.
Robin BN, Chaput CD, Zeitouni S, Rahm MD, Zerris VA, Sampson HW. Cytokine-mediated inflammatory reaction following posterior cervical decompression and fusion associated with recombinant human bone morphogenetic protein-2: a case study. Spine (Phila Pa 1976). 2010;35:E1350–4.
Singh K, Dumonski M, Stanley T, Ponnappan R, Phillips FM. Repeat use of human recombinant bone morphogenetic protein-2 for second level lumbar arthrodesis. Spine (Phila Pa 1976). 2011;36:192–6.
Carreon LY, Glassman SD, Brock DC, Dimar JR, Puno RM, Campbell MJ. Adverse events in patients re-exposed to bone morphogenetic protein for spine surgery. Spine (Phila Pa 1976). 2008;33:391–3.
McGovern JA, Griffin M, Hutmacher DW. Animal models for bone tissue engineering and modelling disease. Dis Model Mech. 2018;11:dmm033084.
Scott MA, Levi B, Askarinam A, Nguyen A, Rackohn T, Ting K, et al. Brief review of models of ectopic bone formation. Stem Cells Dev. 2012;21:655–67.
Scherer F, Schillinger U, Putz U, Stemberger A, Plank C. Nonviral vector loaded collagen sponges for sustained gene delivery in vitro and in vivo. J Gene Med. 2002;4:634–43.
Howell TH, Fiorellini J, Jones A, Alder M, Nummikoski P, Lazaro M, et al. A feasibility study evaluating rhBMP-2/absorbable collagen sponge device for local alveolar ridge preservation or augmentation. Int J Periodontics Restorative Dent. 1997;17:124–39.
Acknowledgements
We thank Yaran Zang for providing technical assistance and professor Young Kim (Dept. of oral pathology, Chonnam National University) for histology analyses. This study was supported by the National Research Foundation of Korea (NRF) Grants funded by the Korea government (MSIP) (No. 2019R1A5A2027521, 2012K001392).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All animal studies were reviewed and approved by the Animal Ethics Committee of Chonnam National University (CNU IACUC-YB-2017-73).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Son, HJ., Lee, M.N., Kim, Y. et al. Bone Generation Following Repeated Administration of Recombinant Bone Morphogenetic Protein 2. Tissue Eng Regen Med 18, 155–164 (2021). https://doi.org/10.1007/s13770-020-00290-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-020-00290-4