Abstract
Background:
Preterm labor is a leading risk factor for neonatal death and long-term impairment and linked closely with inflammation. Non-obstetric surgery is occasionally needed during pregnancy and the anesthetic drugs or surgery itself can give rise to inflammation. Here, we examined the influence of propofol pretreatment on the expression of cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) after lipopolysaccharide (LPS) stimulation. In addition, we evaluated the expression of pro-inflammatory cytokines and nuclear factor kappa B (NF-κB).
Methods:
Human amnion-derived WISH cells were used to investigate the effect of propofol on the LPS-induced expression of inflammatory substances involved in preterm labor. For the experiment, WISH cells were pretreated with various concentrations propofol (0.01–10 μg/ml) for 1 h and then treated with LPS (1 μg/ml) for 24 h. Cytotoxicity was evaluated using MTT assay. PGE2 concentration was assessed by ELISA. Protein expressions of COX-2, PGE2 and NF-κB were analyzed by western blotting analysis. RT-PCR was used for analysis of mRNA expression of COX-2, PGE2, interlukin (IL)-1β and tumor necrosis factor (TNF)-α.
Results:
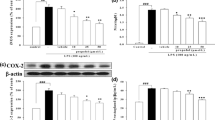
Propofol showed no cytotoxicity on the WISH cells. LPS-induced PGE2 production and COX-2 and PGE2 expression were decreased after propofol pretreatment. Propofol also attenuated the LPS-induced mRNA expression of IL-1β and TNF-α. Moreover, the activation of NF-κB was inhibited by propofol pretreatment on LPS-stimulated WISH cells.
Conclusion:
We demonstrated that propofol suppresses the expression of inflammatory substances enhanced by LPS stimulation. Furthermore, this inhibitory effect of propofol on the inflammatory substance expression is mediated by suppression of NF-κB activation.





Similar content being viewed by others
References
Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–35.
Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84.
Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72.
Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–26.
Upadya M, Saneesh PJ. Anaesthesia for non-obstetric surgery during pregnancy. Indian J Anaesth. 2016;60:234–41.
DiGiulio DB, Romero R, Kusanovic JP, Gómez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57.
Rinaldi SF, Hutchinson JL, Rossi AG, Norman JE. Anti-inflammatory mediators as physiological and pharmacological regulators of parturition. Expert Rev Clin Immunol. 2011;7:675–96.
Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition—a review. Placenta. 2003;24:S33–46.
Teixeira FJ, Zakar T, Hirst JJ, Guo F, Sadowsky DW, Machin G, et al. Prostaglandin endoperoxide-H synthase (PGHS) activity and immunoreactive PGHS-1 and PGHS-2 levels in human amnion throughout gestation, at term, and during labor. J Clin Endocrinol Metab. 1994;78:1396–402.
Slater D, Dennes W, Sawdy R, Allport V, Bennett P. Expression of cyclo-oxygenase types-1 and -2 in human fetal membranes throughout pregnancy. J Mol Endocrinol. 1999;22:125–30.
Allport VC, Pieber D, Slater DM, Newton R, White JO, Bennett PR. Human labour is associated with nuclear factor-kappaB activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal’. Mol Hum Reprod. 2001;7:581–6.
Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034.
Lindström TM, Bennett PR. The role of nuclear factor kappa B in human labour. Reproduction. 2005;130:569–81.
Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–224.
Abboud TK, Zhu J, Richardson M, Peres Da Silva E, Donovan M. Intravenous propofol vs thiamylal-isoflurane for caesarean section, comparative maternal and neonatal effects. Acta Anaesthesiol Scand. 1995;39:205–9.
Higuchi H, Adachi Y, Arimura S, Kanno M, Satoh T. Early pregnancy does not reduce the C(50) of propofol for loss of consciousness. Anesth Analg. 2001;93:1565–9.
Inada T, Kubo K, Kambara T, Shingu K. Propofol inhibits cyclo-oxygenase activity in human monocytic THP-1 cells. Can J Anaesth. 2009;56:222–9.
Kim JD, Ahn BM, Joo BS, Kwon JY, Chung HJ, Yu SB. Effect of propofol on prostaglandin E2 production and prostaglandin synthase-2 and cyclooxygenase-2 expressions in amniotic membrane cells. J Anesth. 2014;28:911–8.
Aarts L, van der Hee R, Dekker I, de Jong J, Langemeijer H, Bast A. The widely used anesthetic agent propofol can replace alpha-tocopherol as an antioxidant. FEBS Lett. 1995;357:83–5.
Vasileiou I, Xanthos T, Koudouna E, Perrea D, Klonaris C, Katsargyris A, et al. Propofol: a review of its non-anaesthetic effects. Eur J Pharmacol. 2009;605:1–8.
Yallampalli C, Garfield RE, Byam-Smith M. Nitric oxide inhibits uterine contractility during pregnancy but not during delivery. Endocrinology. 1993;133:1899–902.
Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol. 1997;272:R441–63.
Kambara T, Inada T, Kubo K, Shingu K. Propofol suppresses prostaglandin E(2) production in human peripheral monocytes. Immunopharmacol Immunotoxicol. 2009;31:117–26.
Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8.
Simmons LE, Rubens CE, Darmstadt GL, Gravett MG. Preventing preterm birth and neonatal mortality: exploring the epidemiology, causes, and interventions. Semin Perinatol. 2010;34:408–15.
Thaxton JE, Nevers TA, Sharma S. TLR-mediated preterm birth in response to pathogenic agents. Infect Dis Obstet Gynecol 2010;2010:378472.
Luo T, Xia Z, Ansley DM, Ouyang J, Granville DJ, Li Y, et al. Propofol dose-dependently reduces tumor necrosis factor-alpha-Induced human umbilical vein endothelial cell apoptosis: effects on Bcl-2 and Bax expression and nitric oxide generation. Anesth Analg. 2005;100:1653–9.
Ledingham MA, Thomson AJ, Greer IA, Norman JE. Nitric oxide in parturition. BJOG. 2000;107:581–93.
Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42.
Di Iulio JL, Gude NM, King RG, Brennecke SP. Human placental and fetal membrane nitric oxide synthase activity before, during and after labour at term. Reprod Fertil Dev. 1995;7:1505–8.
Thomson AJ, Telfer JF, Kohnen G, Young A, Cameron IT, Greer IA, et al. Nitric oxide synthase activity and localization do not change in uterus and placenta during human parturition. Hum Reprod. 1997;12:2546–52.
Marinoni E, Di Iorio R, Villaccio B, Alberini A, Rota F, Cosmi EV. Amniotic fluid nitric oxide metabolite levels and nitric oxide synthase localization in feto-placental tissues are modified in association with human labor. Eur J Obstet Gynecol Reprod Biol. 2000;89:47–54.
Short TG, Aun CS, Tan P, Wong J, Tam YH, Oh TE. A prospective evaluation of pharmacokinetic model controlled infusion of propofol in paediatric patients. Br J Anaesth. 1994;72:302–6.
Allport VC, Slater DM, Newton R, Bennett PR. NF-kappaB and AP-1 are required for cyclo-oxygenase 2 gene expression in amnion epithelial cell line (WISH). Mol Hum Reprod. 2000;6:561–5.
Elliott CL, Allport VC, Loudon JA, Wu GD, Bennett PR. Nuclear factor-kappa B is essential for up-regulation of interleukin-8 expression in human amnion and cervical epithelial cells. Mol Hum Reprod. 2001;7:787–90.
Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–79.
Sun SC. Non-canonical NF-kappaB signaling pathway. Cell Res. 2011;21:71–85.
Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98.
Sun SC. The noncanonical NF-kappaB pathway. Immunol Rev. 2012;246:125–40.
Acknowledgement
This study was supported by Dental Research Institute (PNUDH-DRI 2017-04), Pusan National University Dental Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Statement
There are no animal or human experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yoon, JY., Kim, DW., Ahn, JH. et al. Propofol Suppresses LPS-Induced Inflammation in Amnion Cells via Inhibition of NF-κB Activation. Tissue Eng Regen Med 16, 301–309 (2019). https://doi.org/10.1007/s13770-019-00194-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-019-00194-y