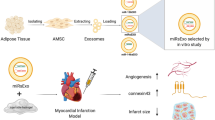
Abstract
BACKGROUND:
The interplay between neurogenesis and angiogenesis is crucial during the development mediated by neuro-angiogenic morphogens. In particular, the angiogenic activity of neuropeptides and their role in tissue regeneration have long been investigated for better understanding of their biological mechanisms and further applications. However, there have been few studies for direct comparison of angiogenic activities of neuropeptides for in vitro and in vivo models. In this study, we report that direct comparison of the angiogenic activities of neuropeptide Y, secretoneurin, and substance P (SP) immobilized on hydrogels in in vitro and in vivo experiments.
METHODS:
A hyaluronic acid-based hydrogel is prepared by utilizing acrylated hyaluronic acid and thiolated peptides as a crosslinker and angiogenic factors, respectively. Angiogenic activities of three neuropeptides are evaluated not only by in vitro angiogenic and gene expression assays, but also by an in vivo chronic myocardial infarction model.
RESULTS:
The comparison of in vitro angiogenic activities of three peptides demonstrates that the SP-immobilized hydrogel shows a higher degree of cell network formation and angiogenic-specific genes than those of the other peptides and the control case. In addition, a three-dimensional angiogenic assay illustrates that more sprouting is observable in the SP group. Evaluation of regenerative activity in the chronic myocardial infarction model reveals that all three peptide-immobilized hydrogels induce increased cardiac function as well as structural regeneration. Among all the cases, the SP group provided the highest regenerative activity both in vitro and in vivo.
CONCLUSION:
In our comparison study, the SP-immobilized hydrogel shows the highest angiogenic activity and tissue regeneration among the test groups. This result suggests that nerve regeneration factors help angiogenesis in damaged tissues, which also highlights the importance of the neuro-angiogenic peptides as an element of tissue regeneration.







Similar content being viewed by others
References
Rouwkema J, Khademhosseini A. Vascularization and angiogenesis in tissue engineering: beyond creating static networks. Trends Biotechnol. 2016;34:733–45.
Zachary I, Morgan RD. Therapeutic angiogenesis for cardiovascular disease: biological context, challenges, prospects. Heart. 2011;97:181–9.
Goncalves LM. Angiogenic growth factors: potential new treatment for acute myocardial infarction? Cardiovasc Res. 2000;45:294–302.
Testa U, Pannitteri G, Condorelli GL. Vascular endothelial growth factors in cardiovascular medicine. J Cardiovasc Med. 2008;9:1190–221.
Jain M, LoGerfo FW, Guthrie P, Pradhan L. Effect of hyperglycemia and neuropeptides on interleukin-8 expression and angiogenesis in dermal microvascular endothelial cells. J Vasc Surg. 2011;53:1654–60.
Lee EW, Michalkiewicz M, Kitlinska J, Kalezic I, Switalska H, Yoo P, et al. Neuropeptide Y induces ischemic angiogenesis and restores function of ischemic skeletal muscles. J Clin Invest. 2003;111:1853–62.
Kim JH, Jung Y, Kim BS, Kim SH. Stem cell recruitment and angiogenesis of neuropeptide substance P coupled with self-assembling peptide nanofiber in a mouse hind limb ischemia model. Biomaterials. 2013;34:1657–68.
Lv T, Liang W, Li L, Cui X, Wei X, Pan H, et al. Novel calcitonin gene-related peptide/chitosan-strontium-calcium phosphate cement: enhanced proliferation of human umbilical vein endothelial cells in vitro. J Biomed Mater Res B Appl Biomater. 2018. https://doi.org/10.1002/jbm.b.34091.
Yang J, Shi QD, Song TB, Feng GF, Zang WJ, Zong CH, et al. Vasoactive intestinal peptide increases VEGF expression to promote proliferation of brain vascular endothelial cells via the cAMP/PKA pathway after ischemic insult in vitro. Peptides. 2013;42:105–11.
Fischer-Colbrie R, Kirchmair R, Kahler CM, Wiedermann CJ, Saria A. Secretoneurin: a new player in angiogenesis and chemotaxis linking nerves, blood vessels and the immune system. Curr Protein Pept Sci. 2005;6:373–85.
Theurl M, Schgoer W, Albrecht K, Jeschke J, Egger M, Beer AG, et al. The neuropeptide catestatin acts as a novel angiogenic cytokine via a basic fibroblast growth factor-dependent mechanism. Circ Res. 2010;107:1326–35.
Albrecht-Schgoer K, Schgoer W, Holfeld J, Theurl M, Wiedemann D, Steger C, et al. The angiogenic factor secretoneurin induces coronary angiogenesis in a model of myocardial infarction by stimulation of vascular endothelial growth factor signaling in endothelial cells. Circulation. 2012;126:2491–501.
Ekstrand AJ, Cao R, Bjorndahl M, Nystrom S, Jonsson-Rylander AC, Hassani H, et al. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc Natl Acad Sci USA. 2003;100:6033–8.
Kohara H, Tajima S, Yamamoto M, Tabata Y. Angiogenesis induced by controlled release of neuropeptide substance P. Biomaterials. 2010;31:8617–25.
Simon-Yarza T, Formiga FR, Tamayo E, Pelacho B, Prosper F, Blanco-Prieto MJ. Vascular endothelial growth factor-delivery systems for cardiac repair: an overview. Theranostics. 2012;2:541–52.
Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5:590–8.
Song M, Jang H, Lee J, Kim JH, Kim SH, Sun K, et al. Regeneration of chronic myocardial infarction by injectable hydrogels containing stem cell homing factor SDF-1 and angiogenic peptide Ac-SDKP. Biomaterials. 2014;35:2436–45.
Kim J, Kim IS, Cho TH, Lee KB, Hwang SJ, Tae G, et al. Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials. 2007;28:1830–7.
Kim J, Kim IS, Cho TH, Kim HC, Yoon SJ, Choi J, et al. In vivo evaluation of MMP sensitive high-molecular weight HA-based hydrogels for bone tissue engineering. J Biomed Mater Res A. 2010;95:673–81.
Del Duca D, Werbowetski T, Del Maestro RF. Spheroid preparation from hanging drops: characterization of a model of brain tumor invasion. J Neurooncol. 2004;67:295–303.
Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs. 2010;11:298–308.
James JM, Mukouyama YS. Neuronal action on the developing blood vessel pattern. Semin Cell Dev Biol. 2011;22:1019–27.
Zhang JH, Badaut J, Tang J, Obenaus A, Hartman R, Pearce WJ. The vascular neural network–a new paradigm in stroke pathophysiology. Nat Rev Neurol. 2012;8:711–6.
Albrecht-Schgoer K, Barthelmes J, Schgoer W, Theurl M, Nardin I, Lener D, et al. Nanoparticular delivery system for a secretoneurin derivative induces angiogenesis in a hind limb ischemia model. J Control Release. 2017;250:1–8.
Tilan JU, Everhart LM, Abe K, Kuo-Bonde L, Chalothorn D, Kitlinska J, et al. Platelet neuropeptide Y is critical for ischemic revascularization in mice. FASEB J. 2013;27:2244–55.
Um JH, Yu JY, Cubon MJ, Park KS. Substance P and Thiorphan synergically enhance angiogenesis in wound healing. Tissue Eng Regen Med. 2016;13:149–54.
Wang Y, Zhang D, Ashraf M, Zhao T, Huang W, Ashraf A, et al. Combining neuropeptide Y and mesenchymal stem cells reverses remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol. 2010;298:H275–86.
Kim J, Park Y, Tae G, Lee KB, Hwang SJ, Kim IS, et al. Synthesis and characterization of matrix metalloprotease sensitive-low molecular weight hyaluronic acid based hydrogels. J Mater Sci Mater Med. 2008;19:3311–8.
Ejaz A, LoGerfo FW, Khabbaz K, Pradhan L. Expression of neuropeptide Y, substance P, and their receptors in the right atrium of diabetic patients. Clin Transl Sci. 2011;4:346–50.
Yoon SJ, Hong S, Fang YH, Song M, Son KH, Son HS, et al. Differential regeneration of myocardial infarction depending on the progression of disease and the composition of biomimetic hydrogel. J Biosci Bioeng. 2014;118:461–8.
Mistrova E, Kruzliak P, Chottova Dvorakova M. Role of substance P in the cardiovascular system. Neuropeptides. 2016;58:41–51.
Wang J, Conboy I. Embryonic versus adult myogenesis: challenging the ‘regeneration recapitulates development’paradigm. J Mol Cell Biol. 2009;2:1–4.
Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of adult exocrine and endocrine pancreas: a possible recapitulation of embryonic development. Diabetes. 1993;42:1715–20.
Imokawa Y, Yoshizato K. Expression of Sonic hedgehog gene in regenerating newt limb blastemas recapitulates that in developing limb buds. 1. Rouwkema J, Khademhosseini A. Vascularization and angiogenesis in tissue engineering: beyond creating static networks. Trends Biotechnol. 2016;34:733–45.
Acknowledgements
This study was supported by the grant from the National Research Foundation of Korea, Republic of Korea (Grant No. 2016-M3A9B6947892) and a Korea University Grant.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
The animal experiment procedures were approved by the institutional animal care and use committee of Korea University College of Medicine (KUIACUC-2015-165).
Additional information
Jaeyeon Lee and Myeongjin Song equally contributed to this paper.
Rights and permissions
About this article
Cite this article
Lee, J., Song, M., Kim, J. et al. Comparison of Angiogenic Activities of Three Neuropeptides, Substance P, Secretoneurin, and Neuropeptide Y Using Myocardial Infarction. Tissue Eng Regen Med 15, 493–502 (2018). https://doi.org/10.1007/s13770-018-0134-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-018-0134-x