Abstract
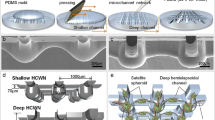
The nervous systems including central and peripheral nervous system have an important role in transmitting signals from brain to organs and tissues. Due to such critical function of nervous system, considerable effort has been tried to establish in vitro nervous model. In this paper, the neuro-spheres networked by the nerve-like structure were created using concave well arrays connected by the hemicylindrical channels. The concave microwells and the hemicylindrical channels were fabricated using the surface tension of viscose liquid PDMS prepolymer for the creation of neuro-spheres-networking (NSN). To investigate the topological effect of the concave-well-hemicylindrical-channel-networking (CWHCN), comparative experiments were conducted on a conventional cylindrical-wells-rectangular-channel-networking (CWRCN). Neuro-progenitor cells from the rat were seeded on the concave well arrays connected by the hemicylindrical channels and cultured for 10 days. Small neuro-pehroids consisting of neurons and glia cells were autonomously formed in the concave microwell arrays. These neuro-spheres were networked by the nerve-like structures formed along the CWHCN. In order to confirm the interconnection of the neurites in the NSN, a calcium imaging experiment was performed to measure the calcium flux along the nerve-like networking. To demonstrate further use of the networked neuro-networking for brain regeneration, we transferred the NSN onto hydrogel maintaining approximately 90% viability, and this model is expected to be used for the regeneration of a damaged brain.
Similar content being viewed by others
References
M Kato-Negishi, Y Tsuda, H Onoe, et al., A neurospheroid network-stamping method for neural transplantation to the brain, Biomaterial, 31, 34 (2010).
YJ Choi, J Park, and S-H Lee, Size-controllable networked neurospheres as a 3D neuronal tissue model for Alzheimer’s disease studies, Biomaterials, 34, 12 (2013).
M Kato-Negishi, Y Morimoto, H Onoe, et al., Millimeter-Sized Neural Building Blocks for 3D Heterogeneous Neural Network Assembly, Advanced healthcare materials, 2, 12 (2013).
C Patrick, Neural tissue engineering, Annals of Biomedical Engineering, 25, 1 (1997).
CE Schmidt and JB Leach, Neural tissue engineering: strategies for repair and regeneration, Annual review of biomedical engineering, 5, 1 (2003).
J Park, E Lim, S Back, et al., Nerve regeneration following spinal cord injury using matrix metalloproteinase-sensitive, hyaluronic acid-based biomimetic hydrogel scaffold containing brain-derived neurotrophic factor, Journal of Biomedical Materials Research Part A, 93, 3 (2009).
SK Kwon and CG Cho, Regeneration of Facial Nerve using Mesenchymal Stem Cells in Facial Nerve Palsy Animal Model, TISSUE ENGINEERING AND REGENERATIVE MEDICINE, 6, 1 (2009).
GRD Evans, Peripheral nerve injury: a review and approach to tissue engineered constructs, The Anatomical Record, 263, 4 (2001).
PG Gross, EP Kartalov, A Scherer, et al., Applications of microfluidics for neuronal studies, Journal of the neurological sciences, 252, 2 (2007).
Y-J Gao and R-R Ji, Chemokines, neuronal-glial interactions, and central processing of neuropathic pain, Pharmacology & therapeutics, 126, 1 (2010).
RJ Miller and PB Tran, Chemokinetics, Neuron, 47, 5 (2005).
SM Kim, SH Kim, CM Kim, et al., Effect of PLGA/DBP scaffolds seeded OECs and SCs on the proliferation and differentiation of BMSCs, Tissue Eng Regen Med, 4, 4 (2007).
JB Recknor, DS Sakaguchi, and SK Mallapragada, Directed growth and selective differentiation of neural progenitor cells on micropatterned polymer substrates, Biomaterials, 27, 22 (2006).
F Yang, R Murugan, S Wang, et al., Electrospinning of nano/micro scale poly (L-lactic acid) aligned fibers and their potential in neural tissue engineering, Biomaterials, 26, 15 (2005).
E Kang, GS Jeong, YY Choi, et al., Digitally tunable physicochemical coding of material composition and topography in continuous microfibres, Nature materials, 10, 11 (2011).
E Kang, YY Choi, S-K Chae, et al., Microfluidic Spinning of Flat Alginate Fibers with Grooves for Cell-aligning Scaffolds, Advanced Materials, 24, 31 (2012).
SB Jun, MR Hynd, N Dowell-Mesfin, et al., Low-density neuronal networks cultured using patterned poly-l-lysine on microelectrode arrays, Journal of neuroscience methods, 160, 2 (2007).
W Jang, S Kim, I Lee, et al., Neurogenesis of bone marrow stromal cell using controlled release of butylated hydroxyanisole from PLGA films. Tissue Eng Regen Med, 2, 2 (2005).
HG Sundararaghavan, GA Monteiro, BL Firestein, et al., Neurite growth in 3D collagen gels with gradients of mechanical properties, Biotechnology and bioengineering, 102, 2 (2009).
TC Holmes, S de Lacalle, X Su, et al., Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds, Proceedings of the National Academy of Sciences, 97, 12 (2000).
LF Reichardt and KJ Tomaselli, Extracellular matrix molecules and their receptors: functions in neural development, Annual review of neuroscience, 14, (1991).
TA Kapur and MS Shoichet, Immobilized concentration gradients of nerve growth factor guide neurite outgrowth, Journal of Biomedical Materials Research Part A, 68, 2 (2004).
CR Kothapalli, E van Veen, S de Valence, et al., A highthroughput microfluidic assay to study neurite response to growth factor gradients, Lab on a chip, 11, 3 (2011).
SW Rhee, AM Taylor, CH Tu, et al., Patterned cell culture inside microfluidic devices, Lab on a chip, 5, 1 (2005).
NS Baek, YH Kim, YH Han, et al., Facile photopatterning of polyfluorene for patterned neuronal networks, Soft Matter, 7, 21 (2011).
R Segev, M Benveniste, E Hulata, et al., Long term behavior of lithographically prepared in vitro neuronal networks, Physical review letters, 88, 11 (2002).
R Segev, M Benveniste, Y Shapira, et al., Formation of electrically active clusterized neural networks, Physical review letters, 90, 16 (2003).
T Gabay, E Jakobs, E Ben-Jacob, et al., Engineered selforganization of neural networks using carbon nanotube clusters, Physica A: Statistical Mechanics and its Applications, 350, 2 (2005).
GS Jeong, Y Jun, JH Song, et al., Meniscus induced self organization of multiple deep concave wells in a microchannel for embryoid bodies generation, Lab on a chip, 12, 1 (2012).
GS Jeong, JH Song, AR Kang, et al., Surface Tension-mediated, Concave-microwell Arrays for Large-scale, Simultaneous Production of Homogeneously Sized Embryoid Bodies, Advanced healthcare materials, 2, 1 (2013).
SF Wong, DY No, YY Choi, et al., Concave microwell based size-controllable hepatosphere as a three-dimensional liver tissue model, Biomaterials, 32, 32 (2011).
S-A Lee, YY Choi, D Park, et al., Functional 3D human primary hepatocyte spheroids made by co-culturing hepatocytes from partial hepatectomy specimens and human adiposederived stem cells, PloS one, 7, 12 (2012).
Y Jun, AR Kang, JS Lee, et al., 3D co-culturing model of primary pancreatic islets and hepatocytes in hybrid spheroid to overcome pancreatic cell shortage, Biomaterials, 34, 15 (2013).
F Bahner, EK Weiss, G Birke, et al., Cellular correlate of assembly formation in oscillating hippocampal networks in vitro, Proceedings of the National Academy of Sciences, 108, 35 (2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeong, G.S. Networked neuro-spheres formed by topological attractants for engineering of 3-dimensional nervous system. Tissue Eng Regen Med 11, 297–303 (2014). https://doi.org/10.1007/s13770-014-4047-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-014-4047-z