Abstract
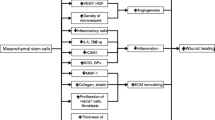
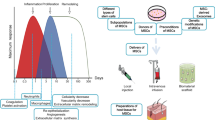
Mesenchymal stem cells (MSCs) exert their therapeutic effects through differentiation into a variety of terminally differentiated cells or the release of paracrine factors. As a source for obtaining MSC, bone marrow has been used chiefly but the exploit of adipose tissue, dental pulp and peripheral blood is also increasing. Previous reports demonstrated that SP mobilizes bone marrow MSC to the circulation to be engaged in tissue repair. SP-mobilized MSC (MSCSP) could be expanded to show the colony forming efficiency and multi-differentiation potential, suggesting the possibility of MSCSP as cellular therapeutics. In this study, we have examined the efficacy of MSCSP in skin wound. MSCSP were topically transplanted to the wound and then, the regeneration was explored in time course. Contrary to vehicle treatment, MSCSP transplantation remarkably reduced the wound size and promoted both of epithelial migration and the recovery of granulation tissue within 7 days after transplantation. Moreover, MSCSPtreated mice showed the increased angiogenesis to make a broadly stretched vessel network. Although it were reported that the transplanted MSCs participated in tissue repair through differentiation, MSCSP was indeed not incorporated into skin cells and localized in dermis layer. As time passes, transplanted MSCSP disappeared rapidly, indicating that MSCSP-induced accelerated healing might be owing to paracrine factors. Collectively, our study revealed that MSCSP can promote skin wound healing, possibly by supplying cytokine and growth factor relating to tissue regeneration. Our results propose MSCSP as a potential therapeutic for inflammatory diseases in addition to cutaneous wound.
Similar content being viewed by others
References
Koc, O. N., Day, J., Nieder, M., Gerson, S. L., Lazarus, H. M., Krivit, W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH), Bone Marrow Transplant, 30, 215–222 (2002).
di Bonzo, L. V., Ferrero, I., Cravanzola, C., Mareschi, K., Rustichell, D., Novo, E., Sanavio, F., Cannito, S., Zamara, E., Bertero, M. et al. Human mesenchymal stem cells as a twoedged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential, Gut, 57, 223–231 (2008).
Coyne, T. M., Marcus, A. J., Woodbury, D., Black, I. B. Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia, Stem Cells, 24, 2483–2492 (2006).
Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D, Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation, Blood, 103, 4619–4621 (2004).
Aggarwal S, Pittenger MF, Human mesenchymal stem cells modulate allogeneic immune cell responses, Blood, 105, 1815–1822 (2005).
Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecifi c mitogenic stimuli, Blood, 99, 3838–3843 (2002).
Ghannam S, Bouffi C, Djouad F, Jorgensen C, Noël D. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Research & Therapy, 1, 2 (2010).
R.M. Gallucci, D.K. Sloan, J.M. Heck, A.R. Murray, S.J. O’Dell, Interleukin 6 indirectly induces keratinocyte migration. J. Invest. Dermatol. 122, 764–772 (2004)
G. Michel, L. Kemeny, R.U. Peter, A. Beetz, C. Ried, P. Arenberger, T. Ruzicka, Interleukin-8 receptor-mediated chemotaxis of normal human epidermal cells, FEBS Lett, 305, 241–243 (1992).
Rameshwar P, Zhu G, Donnelly RJ, Qian J, Ge H, Goldstein KR, Denny TN, Gascón P. The dynamics of bone marrow stromal cells in the proliferation of multipotent hematopoietic progenitors by substance P: an understanding of the effects of neurotransmitter on the differentiating hematopoietic stem cell, J. Neuroimmunol, 121, 22–31 (2001).
Nakamura M, Kawahara M, Morishige N, Chikama T, Nakata K, Nishida T. Promotion of corneal epithelial wound healing in diabetic rats by the combination of a substance P-derived peptide (FGLM-NH2) and insulin-like growth factor-1, Diabetologia, 46, 839 (2003).
An, Y. S., Lee, E., Kang, M. H., Hong, H. S., Kim, M. R., Jang, W. S., Son, Y., Yi, J. Y. Substance P stimulates the recovery of bone marrow after the irradiation, J. Cell Physio, 226, 1204–1213 (2011).
Kang, M. H., Kim, D. Y., Yi, J. Y., Son, Y. Substance-P accelerates intestinal tissue re generation after gamma irradiation- induced damage, Wound Repair Regen, 17, 216–223 (2009).
Jiang MH, Chung EK, Chi GF, Ahn WS, Lim JE, Hong HS, Kim DW, Choi HW, Kim JY, Son YS. Substance P induces M2-type macrophages after spinal cord injury, Neuroreport, 23, 786–792 (2012).
Delgado AV, McManus AT, Chambers JP. Exogenous administration of Substance P enhances wound healing in a novel skin-injury model, Exp Biol Med (Maywood), 230, 271–80 (2005).
Hong HS, Lee J, Lee E, Kwon YS, Lee E, Ahn W, Jiang MH, Kim JC, Son Y. A new role of substance P as an injuryinducible messenger for mobilization of CD29+ stromal-like cells, Nat Med, 15, 425–435 (2009).
Hong HS, Ahn W, Son Y. CD45+/CD11b+ monocytes are required for mesenchymal stem cell proliferation in vitro, Tissue Engineering and Regenerative Medicine 11, 1–6 (2014).
M.N.M. Walter, K.T. Wright, H.R. Fuller, S. MacNeil, W.E.B. Johnson. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: An in vitro study of fibroblast and keratinocytes scratch assays, Exp Cell Res, 316, 1271–81 (2010).
Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis, Stem Cells, 25, 2648–2659 (2007).
Le Blanc, K., Frassoni, F., Ball, L., Locatelli, F., Roelofs, H., Lewis, I., Lanino, E., Sundberg, B., Bernardo, M. E., Remberger, M. et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study, Lancet, 371, 1579–1586 (2008).
S. Kern, H. Eichler, J. Stoeve, H. Kluter, K. Bieback, Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells, 24, 1294–1301 (2006)
Z. Miao, J. Jin, L. Chen, J. Zhu, W. Huang, J. Zhao, H. Qian, X. Zhang, Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells, Cell Biol. Int, 30, 681–687 (2006).
Pittenger MF, Mackay AM, Beck SC et al. Multilineage potential of adult human mesenchymal stem cells, Science, 284, 143–147 (1999).
Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M, Takimoto R et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion, Blood, 106, 756–763 (2005).
Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart, Circulation, human hepatocytes without fusion, Blood, 105, 93–98 (2002).
Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains, Proc Natl Acad Sci USA, 96, 10711–10716 (1999).
Kondo T, Johnson SA, Yoder MC Romand R, Hashino E. Sonic hedgehog and retinoic acid synergistically promote sensory fate specification from bone marrow derived pluripotent stem cells, Proc Natl Acad Sci USA, 102, 4789–4794 (2005).
S. Bajada, P.E. Harrison, B.A. Ashton, V.N. Cassar-Pullicino, N. Ashammakhi, J.B. Richardson. Successful treatment of refractory tibial nonunion using calcium sulphate and bone marrow stromal cell implantation, J. Bone Joint Surg. Br, 89, 1382–1386 (2007).
Z.X. Zhang, L.X. Guan, K. Zhang, Q. Zhang, L.J. Dai. A combined procedure to deliver autologous mesenchymal stromal cells to patients with traumatic brain injury, Cytotherapy, 10, 134–139 (2008)
O.Y. Bang, J.S. Lee, P.H. Lee, G. Lee. Autologous mesenchymal stem cell transplantation in stroke patients, Ann. Neurol. 57, 874–882 (2005).
O. Ringdén, M. Uzunel, I. Rasmusson, M. Remberger, B. Sundberg, H. Lönnies, H.U. Marschall, A. Dlugosz, A. Szakos, Z. Hassan, B. Omazic, J. Aschan, L. Barkholt, K. Le Blanc, Mesenchymal stem cells for treatment of therapy-resistant graftversus-host disease, Transplantation, 81, 1390–1397 (2006).
S.L. Chen, W.W. Fang, F. Ye, Y.H. Liu, J. Qian, S.J. Shan, J.J. Zhang, R.Z. Chunhua, L.M. Liao, S. Lin, J.P. Sun. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction, Am. J. Cardiol, 94, 92–95 (2004).
M. Sasaki, R. Abe, Y. Fujita, S. Ando, D. Inokuma, H. Shimizu, Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type, J. Immunol, 180, 2581–2587 (2008).
Liu, H., Kemeny, D. M., Heng, B. C., Ouyang, H. W., Melendez, A. J. and Cao, T. The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells, J. Immunol, 176, 2864–2871 (2006).
Liwen Chen, Edward E. Tredget, Philip Y. G. Wu, Yaojiong Wu. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing, PLoS ONE, 3, e1886 (2008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hong, H.S., Son, Y. Substance-p-mobilized mesenchymal stem cells accelerate skin wound healing. Tissue Eng Regen Med 11, 483–491 (2014). https://doi.org/10.1007/s13770-014-0062-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-014-0062-3