Abstract
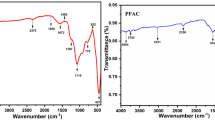
The use of low-cost adsorbent has been investigated in the removal of nickel ions from aqueous solution. The adsorption of nickel is strongly dependent on various parameters such as pH, dosage, contact time and initial concentration of metal. The optimum pH for nickel removal is found to be 7.0. The removal of nickel ions increases with time and attains saturation in about 60–120 min. The experimental data were analyzed with various isotherms such as Freundlich, Langmuir Temkin and Dubinin–Radushkevich adsorption isotherm. The experimental data were fitted into the following kinetic models: Lagergren pseudo-first order, the chemisorption pseudo-second order, Elovich kinetic model, and the intraparticle diffusion model. It was observed that chemisorption pseudo-second-order kinetic model described the sorption process with high coefficients of determination better than any other kinetic models. The results indicate that the second-order model best describes adsorption kinetic data. The adsorbent was characterized; functional group was analyzed by Fourier transform infrared spectroscopy and surface morphology by scanning electron microscope, and energy dispersive X-ray diffraction were analysed.









Similar content being viewed by others
References
Ali I, Asim M, Khan TA (2012) Low cost adsorbents for the removal of organic pollutants from wastewater. J Environ Manage 30(113):170–183. doi:10.1016/j.jenvman.2012.08.028
Al-Meshragi M, Ibrahim HG, Aboabboud MM (2008) Proceedings of the world congress on engineering and computer science 2008, WCECS 2008, October 22–24
Al-Souod K (2012) Kinetics of the adsorption of hexavalent Chromium from aqueous solutions on low cost material. Afr J Pure Appl Chem 6(14):190–197
Alyuz B, Veli S (2009) Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J Hazard Mater 167:482–488
Anoop Krishnan K, Sreejalekshmi KG, Baiju RS (2011) Nickel (II) adsorption onto biomass based activated carbon obtained from sugarcane bagasse pith. Bioresour Technol 102:10239–10247
Borba CE, Guirardello R, Silva EA, Veit MT, Tavares CRG (2006) Removal of nickel (II) ions from aqueous solution by biosorption in a fixed bed column. Experimental and theoretical breakthrough curves. Biochem Eng J 30:184–191
Djomgoue P, Siewe M, Djoufac E, Kenfack P, Njopwouo D (2012) Surface modification of Cameroonian magnetite rich clay with Eriochrome Black T. Application for adsorption of nickel in aqueous solution. Appl Surf Sci 258:7470–7479
Elouear Z, Bouzid J, Boujelben N, Feki M, Montiel A (2008) The use of exhausted olive cake ash (EOCA) as a low cost adsorbent for the removal of toxic metal ions from aqueous solutions. Fuel 87:2582–2589
Garg UK, Kaur MP, Garg VK, Sud D (2008) Removal of Nickel(II) from aqueous solution by adsorption on agricultural waste biomass using a response surface methodological approach. Bioresour Technol 99:1325–1331
Gowda R, Nataraj AG, Manamohan Rao N (2012) Coconut leaves as a low cost adsorbent for the removal of Nickel from Electroplating effluents. Int J Sci Eng Res 2(12):1–5
Gupta VK, Jain CK, Ali I, Sharma M, Saini VK (2003) Removal of cadmium and nickel from wastewater using bagasse fly ash—a sugar industry waste. Water Res 37:4038–4044
Hlihor R-M, Cozma P, Ghinea C, Pavel VL, Robu BM, Gavrilescu M (2010) Thermodynamic study of Cd (II) sorption on soil. Lucr Stiint 53:2
Ho Y-S (2006) Review of second-order models for adsorption systems. J Hazard Mater B136:681–689
Ho YS, McKAY G (1998) A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Trans IChemE 76:Part B
Ho YS, McKay G (2002) Application of kinetic models to the sorption of copper(II) on to peat. Adsorpt Sci Technol 20:8
Jain AK, Gupta VK, Bhatnagar A, Suhas (2003) A comparative study of adsorbents prepared from Industrial wastes for removal of dyes. Sep Sci Technol 38(2):463–481
Kannan, Thambidurai S (2008) Comparative studies on the removal of nickel (II) from aqueous solution by using carbon derived from palmyra palm fruit seeds and commercial activated carbon. Int J Sci Technol 1(1):93–107
Kehinde OO, Oluwatoyin TA, Aderonke OO (2009) Comparative analysis of the efficiencies of two low cost adsorbents in the removal of Cr(VI) and Ni(II) from aqueous solution. Afr J Environ Sci Technol 3(11):360–369
Makeswari M, Santhi T (2013) Optimization of preparation of activated carbon from Ricinus communis leaves by microwave-assisted zinc chloride chemical activation: competitive adsorption of Ni2+ ions form aqueous solution. J Chem 2013:1–13
Mittal A, Mittal J, Malviya A, Gupta VK (2009) Adsorptive removal of hazardous anionic dye Congo red from wastewater using waste materials and recovery by desorption. J Colloid Interface Sci 340:16–26
Mittal A, Mittal J, Malviya A, Gupta VK (2010) Removal and recovery of Chrysoidine Y from aqueous solutions by waste materials. J Colloid Interface Sci 344:497–507
Moghadam MR, Nasirizadeh N, Dashti Z, Babanezhad E (2013) Removal of Fe(II) from aqueous solution using pomegranate peel carbon: equilibrium and kinetic studies. Int J Ind Chem 4:19
Repo E, Warchol JK, Kurniawan TA, Sillanpa MET (2010) Adsorption of Co(II) and Ni(II) by EDTA- and/or DTPA-modified chitosan: kinetic and equilibrium modelling. Chem Eng J 161:73–82
Sivakumar P, Palanisamy PN (2009) Adsorption studies of basic red 29 by a non-conventional activated carbon prepared from Euphorbia Antiquorum L. Int J ChemTech Res 1(3):502–510
Srivastava VC, Mall ID, Mishra IM (2009) Competitive adsorption of cadmium(II) and nickel(II) metal ions from aqueous solution onto rice husk ash. Chem Eng Process 48:370–379
Ucer A, Uyanik SF (2006) Aygun, Adsorption of Cu(II), Cd(II), Zn(II), Mn(II) and Fe(III) ions by tannic acid immobilised activated carbon. Sep Purif Technol 47:113–118
Vinoda VTP, Sashidhar RB, Sreedharc B (2010) Biosorption of nickel and total chromium from aqueous solution by gum kondagogu (Cochlospermum gossypium): a carbohydrate biopolymer. J Hazard Mater 178:851–860
Zhao G, Wu X, Tan X, Wang X (2011) Sorption of heavy metal ions from aqueous solutions: a review. Open Colloid Sci J 4:19–31
Zvinowanda CM, Okonkwo JO, Sekhula MM, Agyei NM, Sadiku R (2009) Application of maize tassel for the removal of Pb, Se, Sr, U and V from borehole water contaminated with mine wastewater in the presence of alkaline metals. J Hazard Mater 164:884–891
Acknowledgments
The authors thank the Management and the Principal of A.S.L Pauls College of Engineering and Technology, Coimbatore, Tamilnadu, and Karpagam University, Coimbatore, Tamilnadu, for help in carrying out this research work successfully.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thenmozhi, R., Santhi, T. Characterization of activated Acacia nilotica seed pods for adsorption of Nickel from aqueous solution. Int. J. Environ. Sci. Technol. 12, 1677–1686 (2015). https://doi.org/10.1007/s13762-014-0531-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-014-0531-1