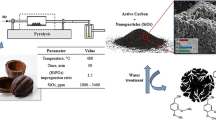
Abstract
Removal of phenol from simulated wastewater was investigated with silver–gold-nanoparticle-modified mango seed shell-activated carbon under batch experiment. The surface properties of the activated carbons were characterized using Fourier transform infrared spectroscopy (FTIR). Adsorption experiment was carried out at ambient temperature to study the effect of contact time, adsorbent dosage, and temperature on phenol adsorption. The equilibrium data were fitted to isotherm model, kinetic model, and intra-particle diffusion models. The maximum removal efficiencies increased from 55.5 to 94.55 and 71.4 to 98.1% for the unmodified and nano-modified activated carbon with increase concentration (50–250 mg/l). The correlation coefficient (R2) Langmuir, Freundlich, and Temkin were 0.3554–0.4563 and 0.2813–0.3191, 0.9150–0.9596 for nanoparticle-modified activated carbon (NCAMSS), as well as 0.5853–0.6598, 0.8159–0.8642, and 0.8159–0.8642, for unmodified activated carbon (UCAMSS). The R2 of the pseudo-first and pseudo-second orders as well as Elovich kinetic models were in the ranges 0.8661–0.9925, 0.8260–0.9942, and 0.6032–0.7505 for NCAMSS as well as 0.4846–0.6032, 0.9567–0.9929, and 0.8842–0.9786, for UCAMSS and modified activated carbon, respectively, The order of fitness/suitability of the models is pseudo-first order > Elovich > pseudo-second order. The intra-particle diffusion model showed that the rate-controlling step is influenced by pore diffusion and that boundary layer diffusion and the adsorption process is heterogeneous, exothermic, and spontaneous. It can be deduced that mango seed shell is a good precursor in the production of activated due to its high yield and good adsorption capacity and the modification of the activated carbon with nanoparticles increased the precursor adsorption properties.
Article Highlights
-
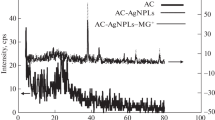
Biosynthesises of silver/gold nanoparticles was successfully used to modify activated carbon developed from mango seed shell.
-
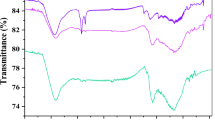
The FTIR of the Mango seed shell AC and the nanoparticle-modified Mango seed shell AC indicated the presence of IR peak ranged from 729.5 to 3902.9 cm−1 and 717.8 to 3985.3 cm−1, respectively.
-
The adsorption results of both nanoparticle-modified and unmodified AC indicated removal efficiencies increased from 55.5 to 94.55% and 71.4 to 98.1%, respectively.
-
Freundlich isotherm is suitable to fit the adsorption of phenol unto activated carbon produced from mango seed shell.
-
The ΔH° values gotten were negative which varied from − 8.8212 to − 22.8643 KJ/mol for the unmodified AC and − 3.694 KJ/mol to − 31.402 KJ/mol for nanoparticle-modified ACs. This showed that the adsorption process of phenol was spontaneous and exothermic.














Similar content being viewed by others
References
Abdelwahab O, Amin N (2013) Adsorption of phenol from aqueous solutions by Luffa cylindrica fibers: kinetics, isotherm and thermodynamic studies. Egypt J Aquat Res 39:215–223
Acosta C, López P, Paniagua G, Garcinuño R, Fernández H (2018) Evaluation of total phenol pollution in water of San Martin Canal from Santiago del Estero, Argentina. J Environ Pollut 236:265–272
Ahsaine HA, Zbair M, Anfar Z (2018) Cationic dyes adsorption onto high surface area ‘almond shell’ activated carbon: kinetics, equilibrium isotherms and surface statistical modeling. Mater Today Chem 8:121–132
Akpen G, Aho I, Mamwan M (2016) Removal of colour from textile wastewater by mango seed endocarp activated carbon. Int J Sci Technol 6(4):756–762
Alabi O, Alade AO, Afolabi TJ (2019) Process optimization of adsorption of Cr(VI) on adsorbent prepared from Bauhinia rufescens pod by Box–Behnken design. Sep Sci Technol. https://doi.org/10.1080/01496395.2019.1577436
Alencar WS, Acayanka E, Lima EC, Royer B, de Souza FE, Lameira J, Alves CN (2012) Application of Mangifera indica (mango) seeds as a biosorbent for removal of Victazol Orange 3R dye from aqueous solution and study of the biosorption mechanism. Chem Eng J 209:577–588
Allen SJ, Whitten L, McKay G (2008) The production and characterisation of activated carbons: a review. Dev Chem Eng Miner Process 6(5):231–261
Al-Othman ZA, Ali R, Naushad M (2012) Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: adsorption kinetics, equilibrium and thermodynamic studies. Chem Eng J 184:238–247
Al-Qodah Z, Shawabkah R (2009) Production and characterization of granular activated carbon from granular sludge. Braz J Chem Eng 26(1):127–136
Amri N, Zakaria R, Abu Bakar M (2009) Adsorption of phenol using adsorbent from waste tyres. Pertanika J Sci Technol 17:371–380
Ashanendu M, Paramartha M, Sudip K (2018) Removal of phenol from aqueous solution using activated carbon from coconut coir. IOSR J Eng (IOSRJEN) 8(12):41–55
Bello O, Bello O, Lateef I (2014) Adsorption characteristics of mango leaf (Mangifera indica) as adsorpbent for malachite green dye removal from aqueous solution. Covenant J Phys Life Sci 2:1–13
Bouchelta C, Medjram MS, Bertrand O, Bellat J-P (2008) Preparation and characterization of activated carbon from date stones by physical activation with steam. J Anal Appl Pyrolysis 82:70–77
Bruce RM, Santodonato J, Neal MW (1987) Summary review of the health effects associated with phenol. Toxicol Ind Health 3:535–568
Bulgariu L, Bulgariu D, Macoveanu M (2011) Adsorptive performances of alkaline treated peat for heavy metal removal. Sep Sci Technol 46:1023–1033
Bullut Y, Tez Z (2007) Adsorption studies on ground shells of hazelnut and almond. J Hazard Mater 149:35–41
Cheung W, Szeto Y, McKay G (2007) Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour Technol 98:2897–2904
Das B, Mondal NK, Bhaumik R, Ray P (2014) Insight into adsorption equilibrium, kinetics and thermodynamics of lead onto alluvial soil. Int J Environ Sci Technol 11:1101–1114
Davila-Jimenez MM, Elizalde-Gonzalez MP, Hernandez-Montoya V (2009) Performance of mango seed adsorbents in the adsorption of anthraquinone and azo acid dyes in single and binary aqueous solutions. Bioresour Technol 100:6199–6206
Dejene K, Siraj K, Kitte S (2016) Kinetic and thermodynamic study of phenol removal from water using activated carbon synthesizes from Avocado kernel seed. Int Lett Nat Sci 54:42–57
Dieguez-Santana K, Villegas-Aguilar P, Le-Thi-Thu H, Castillo-Garit J, Casañola-Martin GM (2016) Prediction of acute toxicity of phenol derivatives using multiple linear regression approach for Tetrahymena pyriformis contaminant identification in a median-size database. Chemosphere 165:434–441
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085
Ejikeme PC, Ebere MO, Gloria N (2014) Equilibrium, kinetics and thermodynamic studies on basic dyes adsorption using composite activated carbon. Int J Tech Res Appl 2:96–103
Elkady MF, Ibrahim AM, Abd El-Latif MM (2011) Assessment of the adsorption kinetics, equilibrium and thermodynamic for the potential removal of reactive red dye using eggshell biocomposite beads. Desalination 278:412–423
Fegousse A, El Gaidoumi A, Miyah Y, El Mountassir R, Lahrichi A (2019) Pineapple bark performance in dyes adsorption: optimization by the central composite design. J Chem. https://doi.org/10.1155/2019/3017163
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Ge M, Wang X, Du M, Liang G, Hu G, Alam JSM (2019) Adsorption analyses of phenol from aqueous solutions using magadiite modified with organo-functional groups: kinetic and equilibrium studies. Materials 12:96. https://doi.org/10.3390/ma12010096
Gumus R, Okpeku I (2015) Production of activated carbon and characterization from snail shell waste (Helix pomatia). Adv Chem Eng Sci 5:51–61
Gupta A, Abdu Rafe M (2013) Removal of phenol from wastewater using mango peel. Int J Eng Tech Res 1:58–62
Hamdaoui M, Hadri M, Bencheqroun Z, Draoui K, Nawdali M, Zaitan H, Barhoun A (2018) Improvement of phenol removal from aqueous medium by adsorption on organically functionalized Moroccan stevensite. J Mater Environ Sci 9(4):1119–1128
Krishnaveni J, Renugadevi N (2012) Kinetic studies on biosorption of methyl violet dye using blue green algae. Global J Sci Front Res Chem 12(3):1–9
Kulkarni SJ, Kaware DJP (2013) Review on research for removal of phenol from waste-water. Int J Sci Res Publ 3:1–4
Kumar KV, Kumaran A (2005) Removal of methylene blue by mango seed kernel powder. Biochem Eng J 27:83–93
Kushwaha A, Gupta N, Chattopadhyaya M (2014) Removal of cationic methylene blue and malachite green dyes from aqueous solution by waste materials of Daucus carota. J Saud Chem Soc 18:200–207
Lekene NRB, Kouoh SPMA, Ndi NJ, Kouotou D, Belibi BPD, Ketcha MJ (2015) Kinetics and equilibrium studies of the adsorption of phenol and methylene blue onto cola nut shell based activated carbon. Int J Cur Res Rev 7(9):1–9
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38:2221–2295
Lateef A, Ojo S, Folarin B, Gueguim-Kana E, Beukes L (2016) Kolanut (Cola nitida) mediated synthesis of silver-gold alloy nanoparticles: antifungal, catalytic, larvicidal and thrombolytic applications. J Cluster Sci 15(5):1561–1577
Lin L, Zhai S-R, Xiao Z-Y, Song Y, An QD, Song XW (2013) Dye adsorption of mesoporous activated carbons produced from NaOH-pretreated rice husks. Bioresour Technol 136:437–443
Ma Y, Gao N, Chu W, Li C (2013) Removal of phenol by powdered activated carbon adsorption. Front Environ Sci Eng 7:158–165
Meireles CS, Rodrigues-Filho GF, Jr MF, Cerqueira DA, Assunção RMN, Ribeiro P, Poletto MZ (2010) Characterization of asymmetric membranes of cellulose acetate from biomass: newspaper and mango seed. Carbohydr Polym 80:954–961
Okeowo IO, Balogun EO, Alade AO, Afolabi TJ, Farombi AG (2017) Optimization of conditions for the production of activated carbon from mango seed shell using barium chloride as the activating agent. In: Paper presented at the 7th international biotechnology conference, exhibition and workshop. Covenant University, Ota
Olajire A, Abidemi J, Lateef A, Benson N (2017) Adsorptive desulphurization of model oil by Ag nanoparticles-modified activated carbon prepared from brewer’s spent grains. J Environ Chem Eng 5:147–159
Olufemi BA, Otolorin F (2017) Comparative adsorption of crude oil using mango (Mangnifera indica) shell and mango shell activated carbon. Environ Eng Res 22(4):384–392. https://doi.org/10.4491/eer.2017.011
Ozer A, Dursun G (2007) Removal of methylene blue from aqueous solution by dehydrated wheat bran carbon. J Hazard Mater 146:262–269
Rai M, Shahi G, Meena V, Meena R, Chakraborty S, Singh R et al (2016) Removal of hexavalent chromium Cr(VI) using activated carbon prepared from mango kernel activated with H3PO4. Resour Efficient Technol 2:63–70
Ramachandran P, Vairamuthu R, Ponnusamy S (2011) Adsorption isotherms, kinetics, thermodynamics and desorption studies of reactive orange16 on activated carbon derived from Ananas comosus (L.) Carbon. ARPN J Eng Appl Sci 6(11):15–26
Rani S, Sud D (2015) Effect of temperature on adsorption-desorption behaviour of triazophos in Indian soils. Plant Soil Environ 61:36–42
Rincon-Silva N, Moreno-Pirajan J, Giraldo L (2016) Equilibrium, kinetics and thermodynamics study of phenols adsorption onto activated carbon obtained from lignocellulosic material (Eucalyptus globulus labill seed). Adsorption 22:33–48
Shirzad-Siboni M, Jafari S, Farrokhi M, Yang J (2013) Removal of phenol from aqueous solutions by activated red mud: equilibrium and kinetics studies. Environ Eng Res 18(4):247–252
Simha P, Banwasi P, Mathew M, Ganesapillai M (2016) Adsorptive resource recovery from human urine: system design, parametric considerations and response surface optimization. Proc Eng 148:779–786
Srivastava V, Swamy MM, Mall I, Prasad B, Mishra I (2006) Adsorptive removal of phenol by bagasse fly ash and activated carbon: equilibrium, kinetics and thermodynamics. Colloids Surf 272:89–104
Tchounwou P, Yedjou C, Patlolla A, Sutton D (2014) Heavy metal toxicity and the environment. Mol Clin Environ Toxicol 101:133–164
Tichapondwa S, Tshemese S, Mhike W (2018) Adsorption of phenol and chromium (VI) pollutants in wastewater using exfoliated graphite. Chem Eng Trans 70:847–852
Tossou G, Ekou L, Ekou T (2019) Adsorption of phenol on carbon based on cactus and banana peel. Aust J Basic Appl Sci 13(1):64–70
Varghese S, Vinod V, Anirudhan T (2004) Kinetics and equilibrium characterization of phenols adsorption onto a novel activated carbon in water treatment. Indian J Chem Technol 11:825–833
Veli S, Alyuz B (2007) Adsorption of copper and zinc from aqueous solutions by using natural clay. J Hazard Mater 149:226–233
Wallace J (1996) Phenol. In: Kirk–Othmer encyclopaedia of chemical technology, 4th edn. Wiley, New York, pp 592–602
Wang X, Hu Y, Min J, Li S, Deng X, Yuan S et al (2018) Adsorption characteristics of phenolic compounds on graphene oxide and reduced graphene oxide: a batch experiment combined theory calculation. Appl Sci 8:1–13
Williams PT, Reed AR (2003) Pre-formed activated carbon matting derived from the pyrolysis of biomass natural fibre textile waste. J Anal Appl Pyrolysis 70(2):563–577
Weber WJ, Morris JC (1963) Kinetics of adsorption carbon from solutions. J Sanit Eng Div 89:31–60
Zbair M, Bottlinger M, Brahmi R, Ainassaari A, Pirilä M, Drif A, Keiski RL, Ojala S, Bensite M (2017) Toward new benchmark adsorbents: preparation and characterization of activated carbon from argan nut shell for bisphenol A removal. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-0634-6
Zbair M, Ahsaine HA, Anfar Z (2018a) Porous carbon by microwave assisted pyrolysis: an effective and low-cost adsorbent for sulfamethoxazole adsorption and optimization using response surface methodology. J Clean Prod. https://doi.org/10.1016/j.jclepro.2018.08.155
Zbair M, Bottlinger M, Ainassaari K, Ojala S, Stein O, Keiski RL, Bensitel M, Brahmi R (2018b) Hydrothermal carbonization of argan nut shell: functional mesoporous carbon with excellent performance in the adsorption of bisphenol A and diuron. Waste Biomass Valoriz. https://doi.org/10.1007/s12649-018-00554-0
Zbair M, Anfar Z, Ahsaine HA, Khallok H (2019) Kinetics, equilibrium, statistical surface modeling and cost analysis of paraquat removal from aqueous solution using carbonated jujube seed. RSC Adv 9:1084–1094
Zhang J (2013) Phenol removal from water with potassium permanganate modified granular activated carbon. J Environ Prot 4:411–417
Zhong Y (2017) Analysis of congo red adsorption capability of the Mn-modified diatomite. Chem Eng Trans 62:25–30
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okeowo, I.O., Balogun, E.O., Ademola, A.J. et al. Adsorption of Phenol from Wastewater Using Microwave-Assisted Ag–Au Nanoparticle-Modified Mango Seed Shell-Activated Carbon. Int J Environ Res 14, 215–233 (2020). https://doi.org/10.1007/s41742-020-00244-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-020-00244-7