Abstract
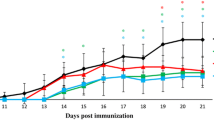
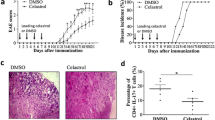
Multiple sclerosis (MS) is the most common inflammatory disorder of the central nervous system (CNS). Kombucha is produced by the fermentation of sugared tea with a symbiotic culture of bacteria and yeasts. This research was designed to reveal the therapeutic impact and the molecular and cellular processes determining the effect of kombucha on MS alleviation in an experimental autoimmune encephalomyelitis (EAE). The EAE was induced using myelin oligodendrocyte glycoprotein (MOG35–55) peptide emulsified in CFA and injected subcutaneously over two flank areas in C57BL/6 mice. In addition, pertussis toxin was injected intraperitoneally and repeated 48 h later. Treatment groups were received three different doses of kombucha (K1: low dose, K2: medium dose and K3: high dose) to obtain a maximum protection. Clinical scores and other criteria were followed daily for the 25 days. At the end of the course, T-helper-related cytokines (IFN-γ, IL-17, IL-4, and TGF-β) were measured through ELISA. Moreover, nitric oxide (NO) concentration in spinal cord tissue was detected. The severity of disease on the peak of disease in K1, K2, and K3 groups were 3.4 ± 0.18 and 2.6 ± 0.18 and 2 ± 0.14 respectively, compared to the CTRL group with 4.5 ± 0.19 (p < 0.001). Kombucha increased production of interleukin IL-4 (K1 = 95 ± 5, K2 = 110 ± 10, K3 = 115 ± 5 and CTRL = 65 ± 5; p < 0.05) and TGF-β (K1 = 1750 ± 80, K2 = 2050 ± 65, K3 = 2200 ± 75 and CTRL = 850 ± 85; p < 0.001) but concurrently resulted in a remarkable reduction in the production of IFN-γ (K1 = 950 ± 70, K2 = 890 ± 65, K3 = 850 ± 85 and CTRL = 3850 ± 115; p < 0.001) and IL-17 (K1 = 1250 ± 75, K2 = 1050 ± 90, K3 = 970 ± 80 and CTRL = 6450 ± 125; p < 0.001). Moreover, NO concentration in spinal cord tissue in the treatment groups was significantly less than the control group (K1: 35.42 ± 2.1, K2 = 31.21 ± 2.2, K3 = 28.24 ± 2.6 and CTRL = 45.25 ± 2.7; p < 0.05). These results supported that kombucha could reduce the severity of disease in an EAE model through motivating polarization of CD4+ T cells by induction of IL-4 and TGF-β as well as inhibition of IFN-γ and IL-17.



Similar content being viewed by others
References
Dendrou CA, Fugger L, Friese MA (2015) Immunopathology of multiple sclerosis. Nat Rev Immunol 15(9):545–558
Namjooyan F, Ghanavati R, Majdinasab N, Jokari S, Janbozorgi M (2014) Uses of complementary and alternative medicine in multiple sclerosis. J Tradit Complement Med 4(3):145–152
Ransohoff RM (2012) Animal models of multiple sclerosis: the good, the bad and the bottom line. Nat Neurosci 15:1074
Petermann F, Korn T (2011) Cytokines and effector T cell subsets causing autoimmune CNS disease. FEBS Lett 585(23):3747–3757
Yadav V, Shinto L, Bourdette D (2010) Complementary and alternative medicine for the treatment of multiple sclerosis. Expert Rev Clin Immunol 6(3):381–395
Olsson T, Barcellos LF, Alfredsson L (2017) Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol 13(1):25–36
Sanoobar M, Eghtesadi S, Azimi A, Khalili M, Khodadadi B, Jazayeri S et al (2015) Coenzyme Q10 supplementation ameliorates inflammatory markers in patients with multiple sclerosis: a double blind, placebo, controlled randomized clinical trial. Nutritional neuroscience. 18(4):169–176
Ljubisavljevic S, Stojanovic I, Pavlovic D, Milojkovic M, Vojinovic S, Sokolovic D et al (2012) Correlation of nitric oxide levels in the cerebellum and spinal cord of experimental autoimmune encephalomyelitis rats with clinical symptoms. Acta Neurobiol Exp (Wars) 72(1):33–39
Bando Y (2015) Myelin morphology and axon pathology in demyelination during experimental autoimmune encephalomyelitis. Neural Regener Res 10(10):1584–1585
Muili KA, Gopalakrishnan S, Meyer SL, Eells JT, Lyons JA (2012) Amelioration of experimental autoimmune encephalomyelitis in C57BL/6 mice by photobiomodulation induced by 670 nm light. PLoS ONE 7(1):e30655
Høglund RA, Maghazachi AA (2014) Multiple sclerosis and the role of immune cells. World J Exp Med 4(3):27–37
Salehipour Z, Haghmorad D, Sankian M, Rastin M, Nosratabadi R, Soltan Dallal MM et al (2017) Bifidobacterium animalis in combination with human origin of Lactobacillus plantarum ameliorate neuroinflammation in experimental model of multiple sclerosis by altering CD4+ T cell subset balance. Biomed Pharmacother. 95:1535–1548
Lan M, Tang X, Zhang J, Yao Z (2018) Insights in pathogenesis of multiple sclerosis: nitric oxide may induce mitochondrial dysfunction of oligodendrocytes. Rev Neurosci 29(1):39–53
O’Brien NC, Charlton B, Cowden WB, Willenborg DO (1999) Nitric oxide plays a critical role in the recovery of Lewis rats from experimental autoimmune encephalomyelitis and the maintenance of resistance to reinduction. J Immunol 163(12):6841–6847
Letourneau S, Hernandez L, Faris AN, Spence DM (2010) Evaluating the effects of estradiol on endothelial nitric oxide stimulated by erythrocyte-derived ATP using a microfluidic approach. Anal Bioanal Chem 397(8):3369–3375
Theoharides TC (2009) Luteolin as a therapeutic option for multiple sclerosis. J Neuroinflamm 6:29
Venkatesha SH, Astry B, Nanjundaiah SM, Kim HR, Rajaiah R, Yang Y et al (2016) Control of autoimmune arthritis by herbal extracts and their bioactive components. Asian J Pharm Sci 11(2):301–307
Dufresne C, Farnworth E (2000) Tea, kombucha, and health: a review. Food Res Int 33:409–421
Marsh AJ, O'Sullivan O, Hill C, Ross RP, Cotter PD (2014) Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiol 38:171–178
Gharib OA (2009) Effects of Kombucha on oxidative stress induced nephrotoxicity in rats. Chin Med 4:23
Jayabalan R, Malbaša R, Lončar E, Vitas J, Sathishkumar M (2014) A review on kombucha tea—microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr Rev Food Sci Food Saf 13:538–550
Marzban F, Azizi G, Afraei S, Sedaghat R, Seyedzadeh MH, Razavi A et al (2015) Kombucha tea ameliorates experimental autoimmune encephalomyelitis in mouse model of multiple sclerosis. Food Agric Immunol 26(6):782–793
Haghmorad D, Mahmoudi MB, Mahmoudi M, Rab SZ, Rastin M, Shegarfi H et al (2014) Calcium intervention ameliorates experimental model of multiple sclerosis. Oman Med J 29(3):185–189
Nosratabadi R, Rastin M, Sankian M, Haghmorad D, Tabasi N, Zamani S et al (2016) St. John's wort and its component hyperforin alleviate experimental autoimmune encephalomyelitis through expansion of regulatory T-cells. J Immunotoxicol. 13(3):364–374
Ip FCF, Ng YP, Or TCT, Sun P, Fu G, Li JYH et al (2017) Anemoside A3 ameliorates experimental autoimmune encephalomyelitis by modulating T helper 17 cell response. PLoS ONE 12(7):e0182069
Nosratabadi R, Rastin M, Sankian M, Haghmorad D, Mahmoudi M (2016) Hyperforin-loaded gold nanoparticle alleviates experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells and upregulating regulatory T cells. Nanomed Nanotechnol Biol Med 12(7):1961–1971
Ottum PA, Arellano G, Reyes LI, Iruretagoyena M, Naves R (2015) Opposing roles of interferon-gamma on cells of the central nervous system in autoimmune neuroinflammation. Front Immunol 6:539
Sanvito L, Constantinescu C, Gran B, Hart B (2010) The multifaceted role of interferon-γ in central nervous system autoimmune demyelination. Open Autoimmunity J. 2:151–159
Jolivalt CG, Howard RB, Chen LS, Mizisin AP, Lai C-S (2003) A novel nitric oxide scavenger in combination with cyclosporine A ameliorates experimental autoimmune encephalomyelitis progression in mice. J Neuroimmunol 138(1):56–64
Vazquez-Cabral BD, Larrosa-Perez M, Gallegos-Infante JA, Moreno-Jimenez MR, Gonzalez-Laredo RF, Rutiaga-Quinones JG et al (2017) Oak kombucha protects against oxidative stress and inflammatory processes. Chem Biol Interact 272:1–9
Arellano G, Ottum PA, Reyes LI, Burgos PI, Naves R (2015) Stage-specific role of interferon-gamma in experimental autoimmune encephalomyelitis and multiple sclerosis. Front Immunol 6:492
Imitola J, Chitnis T, Khoury SJ (2005) Cytokines in multiple sclerosis: from bench to bedside. Pharmacol Ther 106(2):163–177
Fernando V, Omura S, Sato F, Kawai E, Martinez NE, Elliott SF et al (2014) Regulation of an autoimmune model for multiple sclerosis in Th2-biased GATA3 transgenic mice. Int J Mol Sci 15(2):1700–1718
Speck S, Lim J, Shelake S, Matka M, Stoddard J, Farr A et al (2014) TGF-beta signaling initiated in dendritic cells instructs suppressive effects on Th17 differentiation at the site of neuroinflammation. PLoS ONE 9(7):e102390
Cardia GFE, Silva-Filho SE, Silva EL, Uchida NS, Cavalcante HAO, Cassarotti LL et al (2018) Effect of Lavender (Lavandula angustifolia) essential oil on acute inflammatory response. Evid Based Complement Altern Med 2018:10
Lehmann HC, Köhne A, Meyer zu Hörste G, Dehmel T, Kiehl O, Hartung H-P et al (2007) Role of nitric oxide as mediator of nerve injury in inflammatory neuropathies. J Neuropathol Exp Neurol 66(4):305–312
Pozza M, Bettelli C, Aloe L, Giardino L, Calza L (2000) Further evidence for a role of nitric oxide in experimental allergic encephalomyelitis: aminoguanidine treatment modifies its clinical evolution. Brain Res 855(1):39–46
Dias DS, Fontes LB, Crotti AE, Aarestrup BJ, Aarestrup FM, da Silva Filho AA et al (2014) Copaiba oil suppresses inflammatory cytokines in splenocytes of C57Bl/6 mice induced with experimental autoimmune encephalomyelitis (EAE). Molecules (Basel, Switzerland) 19(8):12814–12826
Ibiza S, Serrador JM (2008) The role of nitric oxide in the regulation of adaptive immune responses. Inmunología 27(3):103–117
Stanislaus R, Gilg AG, Singh AK, Singh I (2002) Immunomodulation of experimental autoimmune encephalomyelitis in the Lewis rats by Lovastatin. Neurosci Lett 333(3):167–170
La Flamme AC, Patton EA, Bauman B, Pearce EJ (2001) IL-4 plays a crucial role in regulating oxidative damage in the liver during schistosomiasis. J Immunol 166(3):1903–1911
Gharib OA (2009) Effects of kombucha on oxidative stress induced nephrotoxicity in rats. Chin Med 4:23
Funding
The authors would like to thank the authorities in research council of Semnan University of Medical Sciences for their financial support (Grant Numbers 671).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial or commercial conflict of interest. The authors alone are responsible for the content of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Haghmorad, D., Yazdanpanah, E., Sadighimoghaddam, B. et al. Kombucha ameliorates experimental autoimmune encephalomyelitis through activation of Treg and Th2 cells. Acta Neurol Belg 121, 1685–1692 (2021). https://doi.org/10.1007/s13760-020-01475-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-020-01475-3