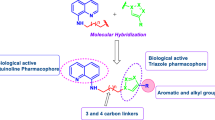
Abstract
Cyclocondensation of 2-chloroquinoline-3-carbaldehydes and 2-thiophenol/2-aminophenols led to the formation of benzo[2,3][1,4]thio- or oxazepino[7,6-b]quinolines. Ugi reaction of the latter compound with various carboxylic acids and isocyanides gave novel oxazepino[7,6-b]quinoline derivatives. All compounds were evaluated for their anti-bacterial and anti-fungal activities. Among them, compounds 4a, 4b and 4d showed moderate to good activity.

Similar content being viewed by others
References
G. Campiani, V. Nacci, I. Fiorini, M.P. De Filippis, A. Garofalo, S.M. Ciani, G. Greco, E. Novellino, D.C. Williams, D.M. Zisterer, M.J. Woods, C. Mihai, C. Manzoni, T. Mennini, J. Med. Chem. 39, 3435 (1996)
I. Fiorini, V. Nacci, S.M. Ciani, A. Garofalo, G. Campiani, L. Savini, E. Novellino, G. Greco, P. Bernasconi, T. Mennini, J. Med. Chem. 37, 1427 (1994)
G. Greco, E. Novellino, I. Fiorini, V. Nacci, G. Campiani, S.M. Ciani, A. Garofalo, P. Bernasconi, T. Mennini, J. Med. Chem. 37, 4100 (1994)
G. DeSarro, A. Chimirri, A. DeSarro, R. Gitto, S. Grasso, M. Zappala, Eur. J. Med. Chem. 30, 925 (1995)
T. MiKi, M. Kori, H. Mabuchi, R. Tozawa, T. Nishimotos, Y. Sugiyama, K. Teshima, H. Yukimasa, J. Med. Chem. 45, 4571 (2002)
F. Bihel, J.L. Kraus, Org. Biomol. Chem. 1, 793 (2003)
F.G. Sulman, Y. Pfeifer, E. Superstine, Arzneimittelforschung 31, 109 (1981)
P.P.M.A. Dols, B.J.B. Folmer, H. Hamersma, C.W. Kuil, H. Lucas, L. Ollero, J.B.M. Rewinkel, P.H.H. Hermkens, Bioorg. Med. Chem. Lett. 18, 1461 (2008)
V.J. Merluzzi, K.D. Hargrave, M. Labadia, K. Grozinger, M. Skoog, J.C. Wu, C.K. Shih, K. Eckner, S. Hattox, J. Adams, A.S. Rosenthal, R. Faanes, R.J. Eckner, R.A. Koup, J.L. Sullivan, Science 250, 1411 (1990)
X.Q. Deng, C.X. Wei, F.N. Li, Z.G. Sun, Z.S. Quan, Eur. J. Med. Chem. 45, 3080 (2010)
R.A. Smits, H.D. Lim, B. Stegink, R.A. Bakker, I.J.P. de Esch, R. Leurs, J. Med. Chem. 49, 4512 (2006)
R. Li, P.S. Farmer, J. Wang, R.J. Boyd, T.S. Cameron, M.A. Quilliam, J.A. Walter, S.E. Howlett, Drug Des. Dis. 12, 337 (1995)
K. Nagarajan, J. David, Y.S. Kulkarni, S.B. Hendi, S.J. Shenoy, P. Upadhyaya, Eur. J. Med. Chem. Chim. Theory 21, 21 (1986)
E.A. Hallinan, T.J. Hagen, S. Tsymbalov, A. Stapelfeld, M.A. Savage, Bioorg. Med. Chem. 9, 1 (2001)
W.J. Van der Burg, Chem. Abstr. 81, 3986 (1974). (DE2347727)
M. Dıaz-Gavilan, F. Rodrıtimeguez-Serrano, J.A. Gomez-Vidal, J.A. Marchal, A. Aranega, M.A. Gallo, A. Espinosa, J.M. Campos, Tetrahedron 60, 11547 (2004)
J.P. Michael, Nat. Prod. Rep. 25, 166 (2008)
J. Marco-Contelles, M.D. Carreiras, The Friedlander Reaction (Lambert Academic, Saarbrucken, Germany, 2010)
J. Marco-Contelles, E. Perez-Mayoral, A. Samadi, M.D. Carreiras, E. Soriano, Chem. Rev. 109, 2652 (2009)
M. Shiri, M.A. Zolfigol, H.G. Kruger, Z. Tanbakouchian, in Friedlander Annulation in the Synthesis of Azaheterocyclic Compounds in Advances in Heterocyclic Chemistry, ed. by A.R. Katritzky (Academic, Oxford, 2011), vol. 185, p 139
P.M.S. Chauhan, S.K. Srivastava, Curr. Med. Chem. 8, 1535 (2001)
Y.L. Chen, K.G. Fang, J.Y. Sheu, S.L. Hsu, C.C. Tzeng, J. Med. Chem. 44, 2374 (2001)
G. Roma, M.D. Braccio, G. Grossi, F. Mattioli, M. Ghia, Eur. J. Med. Chem. 35, 1021 (2000)
Y. Morizawa, T. Okazoc, S.Z. Wang, J. Sasaki, H. Ebisu, M. Nishikawa, H. Shinyyama, J. Fluor. Chem. 109, 83 (2001)
K. Goto, O. Yaoka, T. Oe, PCT Int. Appl. WO 8401711, A1 (1984)
Y. Maruyama, K. Goto, M. Terasawa, Ger. Offen. DE 3010751, (1981)
K. Ukawa, T. Ishiguro, H. Kuriki, A. Nohara, Chem. Pharm. Bull. 33, 4432 (1985)
G.P. Zecchini, I. Torrini, M.P. Paradisi, Heterocycles 26, 2443 (1987)
I. Torrini, G.P. Zecchini, M.P. Paradisi, F. Scazzocchio, Heterocycles 31, 1687 (1990)
S.S. Sonar, S.A. Sadaphal, V.B. Labade, B.B. Shingate, M.S. Shingare, Phosphorus Sulfur Silicon Rel. Elem. 185, 65 (2010)
A. Domling, I. Ugi, Angew. Chem. Int. Ed. 39, 3168 (2000)
A. Domling, Chem. Rev. 106, 17 (2006)
M. Shiri, M.A. Zolfigol, M. Pirveysian, R. Ayazi-Nasrabadi, H.G. Kruger, T. Naicker, I. Mohammadpoor-Baltork, Tetrahedron 68, 6059 (2012)
M.A. Zolfigol, P. Salehi, A. Ghaderi, M. Shiri, Z. Tanbakouchian, J. Mol. Catal. A: Gen. 259, 253 (2006)
M.A. Zolfigol, P. Salehi, A. Ghaderi, M. Shiri, J. Chin. Chem. Soc. 54, 267 (2007)
M.A. Zolfigol, P. Salehi, M. Shiri, A. Ghaderi, Catal. Commun. 8, 1214 (2007)
M.A. Zolfigol, P. Salehi, M. Shiri, T. Rastegar, A. Ghaderi, J. Iran. Chem. Soc. 5, 490 (2008)
S. Moghimi, M. Shiri, M.M. Heravi, H.G. Kruger, Tetrahedron Lett. 54, 6215 (2013)
B. Soleymanifard, M.M. Heravi, M. Shiri, M.A. Zolfigol, M. Rafiee, H.G. Kruger, T. Naicker, F. Rasekhmanesh, Tetrahedron Lett. 53, 3546 (2012)
M. Shiri, Z. Bozorgpour-Savadjani, J. Iran. Chem. Soc. 12, 389 (2015)
S. Sadjadi, M.M. Heravi, Tetrahedron 67, 2707 (2011)
M. Shiri, Chem. Rev. 112, 3508 (2012)
M. Shiri, M.A. Zolfigol, T. Faal-Rastegar, H.G. Kruger, J. Iran. Chem. Soc. 11, 85 (2014)
M. Shiri, S.Z. Mirpour-Marzoni, Z. Bozorgpour-Savadjani, B. Soleymanifard, H.G. Kruger, Monatsh. Chem. 145, 1947 (2014)
O. Meth-Cohn, B. Narine, B. Tarnowski, J. Chem. Soc. Perkin 1, 1520 (1981)
Acknowledgments
We thank Alzahra University and Iran National Science Foundation (INSF) for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hamidi, H., Heravi, M.M., Tajbakhsh, M. et al. Synthesis and anti-bacterial evaluation of novel thio- and oxazepino[7,6-b]quinolines. J IRAN CHEM SOC 12, 2205–2212 (2015). https://doi.org/10.1007/s13738-015-0698-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0698-5