Abstract
A library of 20 novel quinoline-1,2,3-triazole hybrid compounds were synthesized by starting from 8-aminoquinoline to discover new pharmacophores exhibiting antimicrobial activities. Effective and targeted selective biologically active molecules through conjugation of diversely substituted triazoles and 8-aminoquinoline were achieved successfully with 1,4-disubstituted regioisomer product in the 3 + 2 cycloaddition reaction, as expected. All the synthesized compounds were evaluated for antimicrobial activity against different antibacterial and antifungal pathogenic strains. Pleasingly, the compound 9a was found as the most potent against Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterococcus faecalis, Staphylococcus aureus, Streptococcus pneumoniae, Bacillus subtilis, Candida albicans and Candida parapsilosis strains. Preliminary screening results indicated that triazole linked quinoline compounds demonstrate promising antimicrobial activities against Gram (+) and Gram (−) bacterial and fungi strains. Furthermore, the pharmacokinetic properties of the quinoline-1,2,3-triazole hybrid compounds were analyzed to evaluate their potential as drug candidates, which indicated that all compounds are in agreement with Lipinski’s rule of five.

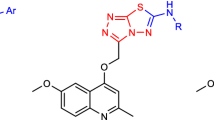
Graphical abstract


Similar content being viewed by others
References
Miller JR, Waldrop GL. Discovery of novel antibacterials. Expert Opin Drug Discov. 2010;5:145–54.
Kong Q, Yang Y. Recent advances in antibacterial agents. Bioorg Med Chem Lett. 2021;35:127799.
Giamarellou H, Poulakou G. Multidrug-resistant gram-negative infections. Drugs. 2009;69:1879–901.
Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century—a clinical super-challenge. N Engl J Med. 2009;360:439–43.
Pokrovskaya V, Baasov T. Dual-acting hybrid antibiotics: a promising strategy to combat bacterial resistance. Expert Opin Drug Discov. 2010;5:883–902.
Vitaku E, Smith DT, Njardarson JT. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J Med Chem. 2014;57:10257–74.
Thomas KD, Adhikari AV, Shetty NS. Design, synthesis and antimicrobial activities of some new quinoline derivatives carrying 1,2,3-triazole moiety. Eur J Med Chem. 2010;45:3803–10.
Zablotskaya A, Segal I, Geronikaki A, Shestakova I, Nikolajeva V, Makarenkova G. N-Heterocyclic choline analogues based on 1,2,3,4-tetrahydro(iso)quinoline scaffold with anticancer and anti-infective dual action. Pharmacol Rep. 2017;69:575–81.
Uddin A, Chawla M, Irfan I, Mahajan S, Singh S, Abid M. Medicinal chemistry updates on quinoline- and endoperoxide-based hybrids with potent antimalarial activity. RSC Med Chem. 2021;12:24–42.
Sun N, Du R-L, Zheng Y-Y, Huang B-H, Guo Q, Zhang R-F, et al. Antibacterial activity of N-methylbenzofuro[3,2-b]quinoline and N-methylbenzoindolo[3,2-b]-quinoline derivatives and study of their mode of action.Eur J Med Chem. 2017;135:1–11.
García E, Coa JC, Otero E, Carda M, Vélez ID, Robledo SM, et al. Synthesis and antiprotozoal activity of furanchalcone–quinoline, furanchalcone–chromone and furanchalcone–imidazole hybrids. Med Chem Res. 2018;27:497–511.
Nathubhai A, Haikarainen T, Koivunen J, Murthy S, Koumanov F, Lloyd M. D. et al. Highly potent and isoform selective dual site binding tankyrase/Wnt signaling inhibitors that increase cellular glucose uptake and have antiproliferative activity. J Med Chem. 2017;60:814–20.
Fouda AM. Halogenated 2-amino-4H-pyrano[3,2-h]quinoline-3-carbonitriles as antitumor agents and structure–activity relationships of the 4-, 6-, and 9-positions. Med Chem Res. 2017;26:302–13.
Pinz MP, Reis AS, de Oliveira RL, Voss GT, Vogt AG, Sacramento M, et al. 7-Chloro-4-phenylsulfonyl quinoline, a new antinociceptive and anti-inflammatory molecule: structural improvement of a quinoline derivate with pharmacological activity. Regul Toxicol Pharmacol. 2017;90:72–7.
Ben Yaakov D, Shadkchan Y, Albert N, Kontoyiannis DP, Osherov N. The quinoline bromoquinol exhibits broad-spectrum antifungal activity and induces oxidative stress and apoptosis in Aspergillus fumigatus. J Antimicrob Chemother. 2017;72:2263–72.
Murugavel S, Jacob Prasanna Stephen CS, Subashini R, AnanthaKrishnan D. Synthesis, structural elucidation, antioxidant, CT-DNA binding and molecular docking studies of novel chloroquinoline derivatives: Promising antioxidant and anti-diabetic agents. J Photochem Photobio B Biol. 2017;173:216–30.
Zhong F, Geng G, Chen B, Pan T, Li Q, Zhang H, et al. Identification of benzenesulfonamide quinoline derivatives as potent HIV-1 replication inhibitors targeting Rev protein. Org Biomol Chem. 2015;13:1792–9.
El Shehry MF, Ghorab MM, Abbas SY, Fayed EA, Shedid SA, Ammar YA. Quinoline derivatives bearing pyrazole moiety: Synthesis and biological evaluation as possible antibacterial and antifungal agents. Eur J Med Chem. 2018;143:1463–73.
Ma X, Zhou W, Brun R. Synthesis, in vitro antitrypanosomal and antibacterial activity of phenoxy, phenylthio or benzyloxy substituted quinolones. Bioorg Med Chem Lett. 2009;19:986–9.
Sanchez JP, Domagala JM, Hagen SE, Heifetz CL, Hutt MP, Nichols JB, et al. Quinolone antibacterial agents. Synthesis and structure-activity relationships of 8-substituted quinoline-3-carboxylic acids and 1,8-naphthyridine-3-carboxylic acids. J Med Chem. 1988;31:983–91.
Gholap AR, Toti KS, Shirazi F, Kumari R, Bhat MK, Deshpande MV, et al. Synthesis and evaluation of antifungal properties of a series of the novel 2-amino-5-oxo-4-phenyl-5,6,7,8-tetrahydroquinoline-3-carbonitrile and its analogues. Bioorg Med Chem. 2007;15:6705–15.
Kharkar PS, Deodhar M, Kulkarni V. Design, synthesis, antifungal activity, and ADME prediction of functional analogues of terbinafine. Med Chem Res. 2008;18:421–32.
Marella A, Tanwar OP, Saha R, Ali MR, Srivastava S, Akhter M, et al. Quinoline: a versatile heterocyclic. Saudi Pharm J. 2013;21:1–12.
Cui S-F, Ren Y, Zhang S-L, Peng X-M, Damu GLV, Geng R-X, et al. Synthesis and biological evaluation of a class of quinolone triazoles as potential antimicrobial agents and their interactions with calf thymus DNA. Bioorg Med Chem Lett. 2013;23:3267–72.
Peng X-M, Cai G-X, Zhou C-H. Recent developments in azole compounds as antibacterial and antifungal agents. Curr Top Med Chem. 2013;13:1963–2010.
Zhang F-F, Gan L-L, Zhou C-H. Synthesis, antibacterial and antifungal activities of some carbazole derivatives. Bioorg Med Chem Lett. 2010;20:1881–4.
Wang Q, Zhang J, Damu GLV, Wan K, Zhang H, Zhou C. Synthesis and biological activities of thio-triazole derivatives as novel potential antibacterial and antifungal agents. Sci China Chem. 2012;55:2134–53.
Zhou C, Cui S, Lv J, Damu GLV, Wang Y. Recent advances in application of thiazole compounds. Sci Sin Chim. 2012;42:1105–31.
Cui S-F, Peng L-P, Zhang H-Z, Rasheed S, Vijaya Kumar K, Zhou C-H. Novel hybrids of metronidazole and quinolones: synthesis, bioactive evaluation, cytotoxicity, preliminary antimicrobial mechanism and effect of metal ions on their transportation by human serum albumin. Eur J Med Chem. 2014;86:318–34.
Parente-Rocha JA, Bailão AM, Amaral AC, Taborda CP, Paccez JD, Borges CL, et al. Antifungal resistance, metabolic routes as drug targets, and new antifungal agents: an overview about endemic dimorphic fungi. Mediators Inflamm. 2017;2017:9870679
Anusionwu CG, Aderibigbe BA, Mbianda XY. Hybrid molecules development: a versatile landscape for the control of antifungal drug resistance: a review. mini-reviews. Med Chem. 2019;19:450–64.
Castaldo N, Givone F, Peghin M, Righi E, Sartor A, Bassetti M. Multidrug-resistant Pseudomonas aeruginosa skin and soft-tissue infection successfully treated with ceftolozane/tazobactam. J Glob Antimicrob Resist. 2017;9:100–2.
Kuti JL, Pettit RS, Neu N, Cies JJ, Lapin C, Muhlebach MS, et al. Microbiological activity of ceftolozane/tazobactam, ceftazidime, meropenem, and piperacillin/tazobactam against Pseudomonas aeruginosa isolated from children with cystic fibrosis. Diagn Microbiol Infect Dis. 2015;83:53–5.
Krstulović L, Stolić I, Jukić M, Opačak-Bernardi T, Starčević K, Bajić M, et al. New quinoline-arylamidine hybrids: synthesis, DNA/RNA binding and antitumor activity. Eur J Med Chem. 2017;137:196–210.
Walsh J, Bell A. Hybrid drugs for malaria. Curr Pharm Des. 2009;15:2970–85.
Muregi FW, Ishih A. Next-generation antimalarial drugs: hybrid molecules as a new strategy in drug design. Drug Dev Res. 2010;71:20–32.
Vandekerckhove S, D’hooghe M. Quinoline-based antimalarial hybrid compounds. Bioorg Med Chem. 2015;23:5098–119.
Gupta V, Datta P. Next-generation strategy for treating drug resistant bacteria: antibiotic hybrids. Indian J Med Res. 2019;149:97.
Alovero F, Nieto M, Mazzieri MR, Then R, Manzo RH. Mode of action of sulfanilyl fluoroquinolones. Antimicrob Agents Chemother. 1998;42:1495–8.
Locher HH, Caspers P, Bruyère T, Schroeder S, Pfaff P, Knezevic A, et al. Investigations of the mode of action and resistance development of cadazolid, a new antibiotic for treatment of Clostridium difficile infections. Antimicrob Agents Chemother. 2014;58:901–8.
Gorityala BK, Guchhait G, Goswami S, Fernando DM, Kumar A, Zhanel GG, et al. Hybrid antibiotic overcomes resistance in P. aeruginosa by enhancing outer membrane penetration and reducing efflux. J Med Chem. 2016;59:8441–55.
Shavit M, Pokrovskaya V, Belakhov V, Baasov T. Covalently linked kanamycin—Ciprofloxacin hybrid antibiotics as a tool to fight bacterial resistance. Bioorg Med Chem. 2017;25:2917–25.
Meunier B. Hybrid molecules with a dual mode of action: dream or reality? Acc Chem Res. 2008;41:69–77.
Ferlin MG, Chiarelotto G, Castagliuolo I. Synthesis and characterization of some N-mannich bases of [1,2,3]triazoloquinolines. J Heterocycl Chem. 2002;39:631–8.
Choi H, Shirley HJ, Hume PA, Brimble MA, Furkert DP. Unexpected direct synthesis of N-Vinyl amides through vinyl azide–enolate [3+2] cycloaddition. Angew Chem Int Ed. 2017;56:7420–4.
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed. 2002;41:2596–9.
Freitas LB, de O, Borgati TF, de Freitas RP, Ruiz ALTG, Marchetti GM, et al. Synthesis and antiproliferative activity of 8-hydroxyquinoline derivatives containing a 1,2,3-triazole moiety. Eur J Med Chem. 2014;84:595–604.
Totobenazara J, Burke AJ. New click-chemistry methods for 1,2,3-triazoles synthesis: recent advances and applications. Tetrahedron Lett. 2015;56:2853–9.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–25.
Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1:337–41.
Veber DF, Johnson SR, Cheng H-Y, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45:2615–23.
Lalitha PSS. Calculation of molecular lipophilicity and drug likeness for few heterocycles. Orient J Chem. 2010;26:135–141.
Manohar S, Khan SI, Rawat DS. Synthesis of 4-aminoquinoline-1,2,3-triazole and 4-aminoquinoline-1,2,3-triazole-1,3,5-triazine hybrids as potential antimalarial agents. Chem Biol Drug Des. 2011;78:124–36.
Woods GL, Washington JA. Antibacterial susceptibility tests: Dilution and disk diffusion method. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH (eds). Manual of Clinical Microbiology, ed 6. Washington DC: American Society for Microbiology, 1995:1327–1341.
Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: general principles and contemporary practices. Clin Infect Dis. 1998;26:973–80.
Acknowledgements
We would like to thank Prof. Dr. Ramazan Altundaş, Prof. Dr. Öztekin Algül and Prof. Dr. Aylin Döğen for valuable discussions and helpful editorial comment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Albayrak, F., Çiçek, M., Alkaya, D. et al. Design, synthesis and biological evaluation of 8-aminoquinoline-1,2,3-triazole hybrid derivatives as potential antimicrobial agents. Med Chem Res 31, 652–665 (2022). https://doi.org/10.1007/s00044-022-02866-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-02866-2