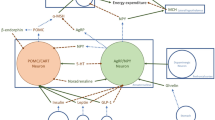
Abstract
Purpose of Review
There is currently a steep rise in the global prevalence of obesity. Pharmaceutical therapy is a valuable component of conservative obesity therapy. Herein, medications currently in the phase of preclinical or clinical testing are reviewed, along with an overview of the mechanisms that regulate energy intake and expenditure. In addition, the current and potential future directions of obesity drug therapy are discussed.
Recent Findings
Although the current arsenal of obesity pharmacotherapy is limited, a considerable number of agents that exert their actions through a variety of pharmacodynamic targets and mechanisms are in the pipeline. This expansion shapes a potential near future of obesity conservative management, characterized by tailored combined therapeutic regimens, targeting not only weight loss but also improved overall health outcomes.
Summary
The progress regarding the elucidation of the mechanisms which regulate the bodily energy equilibrium has led to medications which mimic hormonal adaptations that follow bariatric surgery, in the quest for a “Medical bypass.” These, combined with agents which could increase energy expenditure, point to a brilliant future in the conservative treatment of obesity.
Similar content being viewed by others
Abbreviations
- AgRP:
-
Agouti-related peptide
- BAT:
-
Brown adipose tissue
- BMI:
-
Body mass index
- cGMP:
-
Cyclic guanosine monophosphate
- CVD:
-
Cardiovascular disease
- DACRA:
-
Dual amylin/calcitonin receptor agonists
- DIO:
-
Diet-induced obesity
- DPP4:
-
Dipeptidyl-peptidase-4
- EE :
-
Energy expenditure
- EI:
-
Energy intake
- FDA:
-
Food and Drug Administration
- GCGR :
-
Glucagon receptor
- GHSR1a:
-
Growth hormone secretagogue receptor type 1a
- GIP:
-
Glucose-dependent insulinotropic peptide
- GLP-1:
-
Glucagon-like peptide 1
- GLP1R:
-
Glucagon-like peptide 1 receptor
- GOAT:
-
Ghrelin-O-acyltransferase
- NPY:
-
Neuropeptide Y
- OXM:
-
Oxyntomodulin
- POMC:
-
Proopiomelanocortin
- PYY:
-
Peptide tyrosine-tyrosine
- RYGB:
-
Roux-en-Y gastric bypass
- SCT:
-
Secretin
- SG:
-
Sleeve gastrectomy
- T2DM:
-
Type 2 diabetes mellitus
References
Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32(9):1431–7. https://doi.org/10.1038/ijo.2008.102.
Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74(5):579–84. https://doi.org/10.1093/ajcn/74.5.579.
Dombrowski SU, Knittle K, Avenell A, Araujo-Soares V, Sniehotta FF. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. BMJ. 2014;348:g2646. https://doi.org/10.1136/bmj.g2646.
Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am. 2018;102(1):183–97. https://doi.org/10.1016/j.mcna.2017.08.012.
Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–604. https://doi.org/10.1056/NEJMoa1105816.
Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341–52. https://doi.org/10.1016/S0140-6736(11)60205-5.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. https://doi.org/10.1056/NEJMoa1603827.
Bohula EA, Wiviott SD, McGuire DK, Inzucchi SE, Kuder J, Im K, et al. Cardiovascular safety of lorcaserin in overweight or obese patients. N Engl J Med. 2018;379(12):1107–17. https://doi.org/10.1056/NEJMoa1808721.
Nissen SE, Wolski KE, Prcela L, Wadden T, Buse JB, Bakris G, et al. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: a randomized clinical trial. JAMA. 2016;315(10):990–1004. https://doi.org/10.1001/jama.2016.1558.
Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–95. https://doi.org/10.1038/nature05026.
van den Top M, Spanswick D. Integration of metabolic stimuli in the hypothalamic arcuate nucleus. Prog Brain Res. 2006;153:141–54. https://doi.org/10.1016/S0079-6123(06)53008-0.
Heisler LK, Lam DD. An appetite for life: brain regulation of hunger and satiety. Curr Opin Pharmacol. 2017;37:100–6. https://doi.org/10.1016/j.coph.2017.09.002.
Timper K, Bruning JC. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech. 2017;10(6):679–89. https://doi.org/10.1242/dmm.026609.
Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31(4):506–43. https://doi.org/10.1210/er.2009-0037.
Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104(4):531–43. https://doi.org/10.1016/s0092-8674(01)00240-9.
Zhang W, Bi S. Hypothalamic regulation of brown adipose tissue thermogenesis and energy homeostasis. Front Endocrinol. 2015;6:136. https://doi.org/10.3389/fendo.2015.00136.
Contreras C, Gonzalez F, Ferno J, Dieguez C, Rahmouni K, Nogueiras R, et al. The brain and brown fat. Ann Med. 2015;47(2):150–68. https://doi.org/10.3109/07853890.2014.919727.
Li Y, Schnabl K, Gabler SM, Willershauser M, Reber J, Karlas A, et al. Secretin-activated brown fat mediates prandial thermogenesis to induce satiation. Cell. 2018;175(6):1561–74 e12. https://doi.org/10.1016/j.cell.2018.10.016.
Kokkinos A, Tsilingiris D, le Roux CW, Rubino F, Mantzoros CS. Will medications that mimic gut hormones or target their receptors eventually replace bariatric surgery? Metab Clin Exp. 2019;100:153960. https://doi.org/10.1016/j.metabol.2019.153960.
Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. https://doi.org/10.1056/NEJMoa1411892.
Lau J, Bloch P, Schaffer L, Pettersson I, Spetzler J, Kofoed J, et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem. 2015;58(18):7370–80. https://doi.org/10.1021/acs.jmedchem.5b00726.
Blundell J, Finlayson G, Axelsen M, Flint A, Gibbons C, Kvist T, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017;19(9):1242–51. https://doi.org/10.1111/dom.12932.
O'Neil PM, Birkenfeld AL, McGowan B, Mosenzon O, Pedersen SD, Wharton S, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392(10148):637–49. https://doi.org/10.1016/S0140-6736(18)31773-2.
Goldenberg RM, Steen O. Semaglutide: review and place in therapy for adults with type 2 diabetes. Can J Diabetes. 2019;43(2):136–45. https://doi.org/10.1016/j.jcjd.2018.05.008.
Aroda VR, Ahmann A, Cariou B, Chow F, Davies MJ, Jodar E, et al. Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019;45(5):409–18. https://doi.org/10.1016/j.diabet.2018.12.001.
Jones ES, Nunn N, Chambers AP, Ostergaard S, Wulff BS, Luckman SM. Modified peptide YY molecule attenuates the activity of NPY/AgRP neurons and reduces food intake in male mice. Endocrinology. 2019;160(11):2737–47. https://doi.org/10.1210/en.2019-00100.
van Witteloostuijn SB, Dalboge LS, Hansen G, Midtgaard SR, Jensen GV, Jensen KJ, et al. GUB06-046, a novel secretin/glucagon-like peptide 1 co-agonist, decreases food intake, improves glycemic control, and preserves beta cell mass in diabetic mice. Journal of Peptide Science : an Official Publication of the European Peptide Society. 2017;23(12):845–54. https://doi.org/10.1002/psc.3048.
Ambery P, Parker VE, Stumvoll M, Posch MG, Heise T, Plum-Moerschel L, et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet. 2018;391(10140):2607–18. https://doi.org/10.1016/S0140-6736(18)30726-8.
Larsen AT, Sonne N, Andreassen KV, Gehring K, Karsdal MA, Henriksen K. The dual amylin and calcitonin receptor agonist KBP-088 induces weight loss and improves insulin sensitivity superior to chronic amylin therapy. J Pharmacol Exp Ther. 2019;370(1):35–43. https://doi.org/10.1124/jpet.119.257576.
Gydesen S, Andreassen KV, Hjuler ST, Christensen JM, Karsdal MA, Henriksen K. KBP-088, a novel DACRA with prolonged receptor activation, is superior to davalintide in terms of efficacy on body weight. Am J Physiol Endocrinol Metab. 2016;310(10):E821–7. https://doi.org/10.1152/ajpendo.00514.2015.
Rohrbach K, Thomas MA, Glick S, Fung EN, Wang V, Watson L, et al. Ibipinabant attenuates beta-cell loss in male Zucker diabetic fatty rats independently of its effects on body weight. Diabetes Obes Metab. 2012;14(6):555–64. https://doi.org/10.1111/j.1463-1326.2012.01563.x.
Ma H, Zhang G, Mou C, Fu X, Chen Y. Peripheral CB1 receptor neutral antagonist, AM6545, ameliorates hypometabolic obesity and improves adipokine secretion in monosodium glutamate induced obese mice. Front Pharmacol. 2018;9:156. https://doi.org/10.3389/fphar.2018.00156.
Cluny NL, Vemuri VK, Chambers AP, Limebeer CL, Bedard H, Wood JT, et al. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharmacol. 2010;161(3):629–42. https://doi.org/10.1111/j.1476-5381.2010.00908.x.
Knani I, Earley BJ, Udi S, Nemirovski A, Hadar R, Gammal A, et al. Targeting the endocannabinoid/CB1 receptor system for treating obesity in Prader-Willi syndrome. Mol Metab. 2016;5(12):1187–99. https://doi.org/10.1016/j.molmet.2016.10.004.
Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T, et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012;16(2):167–79. https://doi.org/10.1016/j.cmet.2012.07.002.
Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, Lindberg SO, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42(12):2272–81. https://doi.org/10.2337/dc19-0883.
Pratley R, Amod A, Hoff ST, Kadowaki T, Lingvay I, Nauck M, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394(10192):39–50. https://doi.org/10.1016/S0140-6736(19)31271-1.
Pieber TR, Bode B, Mertens A, Cho YM, Christiansen E, Hertz CL, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):528–39. https://doi.org/10.1016/S2213-8587(19)30194-9.
Vilsboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. https://doi.org/10.1136/bmj.d7771.
Su N, Li Y, Xu T, Li L, Kwong JS, Du H, et al. Exenatide in obese or overweight patients without diabetes: a systematic review and meta-analyses of randomized controlled trials. Int J Cardiol. 2016;219:293–300. https://doi.org/10.1016/j.ijcard.2016.06.028.
Price SL, Bloom SR. Protein PYY and its role in metabolism. Front Horm Res. 2014;42:147–54. https://doi.org/10.1159/000358343.
Ueno H, Yamaguchi H, Mizuta M, Nakazato M. The role of PYY in feeding regulation. Regul Pept. 2008;145(1–3):12–6. https://doi.org/10.1016/j.regpep.2007.09.011.
Ballantyne GH. Peptide YY(1-36) and peptide YY(3-36): part I. Distribution, release and actions. Obes Surg. 2006;16(5):651–8. https://doi.org/10.1381/096089206776944959.
Sam AH, Gunner DJ, King A, Persaud SJ, Brooks L, Hostomska K, et al. Selective ablation of peptide YY cells in adult mice reveals their role in beta cell survival. Gastroenterology. 2012;143(2):459–68. https://doi.org/10.1053/j.gastro.2012.04.047.
Shi YC, Loh K, Bensellam M, Lee K, Zhai L, Lau J, et al. Pancreatic PYY is critical in the control of insulin secretion and glucose homeostasis in female mice. Endocrinology. 2015;156(9):3122–36. https://doi.org/10.1210/en.2015-1168.
Gantz I, Erondu N, Mallick M, Musser B, Krishna R, Tanaka WK, et al. Efficacy and safety of intranasal peptide YY3-36 for weight reduction in obese adults. J Clin Endocrinol Metab. 2007;92(5):1754–7. https://doi.org/10.1210/jc.2006-1806.
Khatib MN, Gaidhane S, Gaidhane AM, Simkhada P, Zahiruddin QS. Ghrelin O acyl transferase (GOAT) as a novel metabolic regulatory enzyme. Journal of Clinical and Diagnostic Research : JCDR. 2015;9(2):LE01–5. https://doi.org/10.7860/JCDR/2015/9787.5514.
Purtell L, Sze L, Loughnan G, Smith E, Herzog H, Sainsbury A, et al. In adults with Prader-Willi syndrome, elevated ghrelin levels are more consistent with hyperphagia than high PYY and GLP-1 levels. Neuropeptides. 2011;45(4):301–7. https://doi.org/10.1016/j.npep.2011.06.001.
Barnett BP, Hwang Y, Taylor MS, Kirchner H, Pfluger PT, Bernard V, et al. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science. 2010;330(6011):1689–92. https://doi.org/10.1126/science.1196154.
Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: similarities and differences. J Diabetes Investig. 2010;1(1–2):8–23. https://doi.org/10.1111/j.2040-1124.2010.00022.x.
Finan B, Ma T, Ottaway N, Muller TD, Habegger KM, Heppner KM, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013;5(209):209ra151. https://doi.org/10.1126/scitranslmed.3007218.
Frias JP, Bastyr EJ 3rd, Vignati L, Tschop MH, Schmitt C, Owen K, et al. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metab. 2017;26(2):343–52 e2. https://doi.org/10.1016/j.cmet.2017.07.011.
Coskun T, Sloop KW, Loghin C, Alsina-Fernandez J, Urva S, Bokvist KB, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab. 2018;18:3–14. https://doi.org/10.1016/j.molmet.2018.09.009.
Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392(10160):2180–93. https://doi.org/10.1016/S0140-6736(18)32260-8.
Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79–101. https://doi.org/10.1146/annurev.nutr.27.061406.093734.
Billington CJ, Briggs JE, Link JG, Levine AS. Glucagon in physiological concentrations stimulates brown fat thermogenesis in vivo. Am J Phys. 1991;261(2 Pt 2):R501–7. https://doi.org/10.1152/ajpregu.1991.261.2.R501.
Kinoshita K, Ozaki N, Takagi Y, Murata Y, Oshida Y, Hayashi Y. Glucagon is essential for adaptive thermogenesis in brown adipose tissue. Endocrinology. 2014;155(9):3484–92. https://doi.org/10.1210/en.2014-1175.
Sanchez-Garrido MA, Brandt SJ, Clemmensen C, Muller TD, DiMarchi RD, Tschop MH. GLP-1/glucagon receptor co-agonism for treatment of obesity. Diabetologia. 2017;60(10):1851–61. https://doi.org/10.1007/s00125-017-4354-8.
Pocai A. Unraveling oxyntomodulin, GLP1’s enigmatic brother. J Endocrinol. 2012;215(3):335–46. https://doi.org/10.1530/JOE-12-0368.
Perakakis N, Kokkinos A, Peradze N, Tentolouris N, Ghaly W, Pilitsi E, et al. Circulating levels of gastrointestinal hormones in response to the most common types of bariatric surgery and predictive value for weight loss over one year: evidence from two independent trials. Metab Clin Exp. 2019;101:153997. https://doi.org/10.1016/j.metabol.2019.153997.
Cohen MA, Ellis SM, Le Roux CW, Batterham RL, Park A, Patterson M, et al. Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab. 2003;88(10):4696–701. https://doi.org/10.1210/jc.2003-030421.
Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG, et al. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes. 2005;54(8):2390–5. https://doi.org/10.2337/diabetes.54.8.2390.
Wynne K, Park AJ, Small CJ, Meeran K, Ghatei MA, Frost GS, et al. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes. 2006;30(12):1729–36. https://doi.org/10.1038/sj.ijo.0803344.
Scott R, Minnion J, Tan T, Bloom SR. Oxyntomodulin analogue increases energy expenditure via the glucagon receptor. Peptides. 2018;104:70–7. https://doi.org/10.1016/j.peptides.2018.04.008.
Ambery PD, Klammt S, Posch MG, Petrone M, Pu W, Rondinone C, et al. MEDI0382, a GLP-1/glucagon receptor dual agonist, meets safety and tolerability endpoints in a single-dose, healthy-subject, randomized, phase 1 study. Br J Clin Pharmacol. 2018;84(10):2325–35. https://doi.org/10.1111/bcp.13688.
Tillner J, Posch MG, Wagner F, Teichert L, Hijazi Y, Einig C, et al. A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: results of randomized, placebo-controlled first-in-human and first-in-patient trials. Diabetes Obes Metab. 2019;21(1):120–8. https://doi.org/10.1111/dom.13494.
Britta Goebel MS, Visentin R, Riz M, Man CD, Cobelli C, Klabunde T. Effects of the novel dual GLP-1R/GCGR agonist SAR425899 on postprandial glucose metabolism in overweight/obese subjects with type 2 diabetes. Diabetes. 2018;67(Supplement 1). https://doi.org/10.2337/db18-72-OR.
Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes. 2009;33(7):786–95. https://doi.org/10.1038/ijo.2009.79.
Bhat VK, Kerr BD, Vasu S, Flatt PR, Gault VA. A DPP-IV-resistant triple-acting agonist of GIP, GLP-1 and glucagon receptors with potent glucose-lowering and insulinotropic actions in high-fat-fed mice. Diabetologia. 2013;56(6):1417–24. https://doi.org/10.1007/s00125-013-2892-2.
Finan B, Yang B, Ottaway N, Smiley DL, Ma T, Clemmensen C, et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21(1):27–36. https://doi.org/10.1038/nm.3761.
Behary P, Tharakan G, Alexiadou K, Johnson N, Wewer Albrechtsen NJ, Kenkre J, et al. Combined GLP-1, oxyntomodulin, and peptide YY improves body weight and glycemia in obesity and prediabetes/type 2 diabetes: a randomized, single-blinded, placebo-controlled study. Diabetes Care. 2019;42(8):1446–53. https://doi.org/10.2337/dc19-0449.
Lutz TA. Control of food intake and energy expenditure by amylin-therapeutic implications. Int J Obes. 2009;33(Suppl 1):S24–7. https://doi.org/10.1038/ijo.2009.13.
Fu W, Patel A, Jhamandas JH. Amylin receptor: a common pathophysiological target in Alzheimer’s disease and diabetes mellitus. Front Aging Neurosci. 2013;5:42. https://doi.org/10.3389/fnagi.2013.00042.
American Diabetes A. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S90–S102. https://doi.org/10.2337/dc19-S009.
Smith SR, Aronne LJ, Burns CM, Kesty NC, Halseth AE, Weyer C. Sustained weight loss following 12-month pramlintide treatment as an adjunct to lifestyle intervention in obesity. Diabetes Care. 2008;31(9):1816–23. https://doi.org/10.2337/dc08-0029.
Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity. 2009;17(9):1736–43. https://doi.org/10.1038/oby.2009.184.
Mack CM, Smith PA, Athanacio JR, Xu K, Wilson JK, Reynolds JM, et al. Glucoregulatory effects and prolonged duration of action of davalintide: a novel amylinomimetic peptide. Diabetes Obes Metab. 2011;13(12):1105–13. https://doi.org/10.1111/j.1463-1326.2011.01465.x.
Mack CM, Soares CJ, Wilson JK, Athanacio JR, Turek VF, Trevaskis JL, et al. Davalintide (AC2307), a novel amylin-mimetic peptide: enhanced pharmacological properties over native amylin to reduce food intake and body weight. Int J Obes. 2010;34(2):385–95. https://doi.org/10.1038/ijo.2009.238.
Hjuler ST, Gydesen S, Andreassen KV, Pedersen SL, Hellgren LI, Karsdal MA, et al. The dual amylin- and calcitonin-receptor agonist KBP-042 increases insulin sensitivity and induces weight loss in rats with obesity. Obesity. 2016;24(8):1712–22. https://doi.org/10.1002/oby.21563.
Hjuler ST, Andreassen KV, Gydesen S, Karsdal MA, Henriksen K. KBP-042 improves bodyweight and glucose homeostasis with indices of increased insulin sensitivity irrespective of route of administration. Eur J Pharmacol. 2015;762:229–38. https://doi.org/10.1016/j.ejphar.2015.05.051.
Marsh DJ, Hollopeter G, Kafer KE, Palmiter RD. Role of the Y5 neuropeptide Y receptor in feeding and obesity. Nat Med. 1998;4(6):718–21. https://doi.org/10.1038/nm0698-718.
Pedrazzini T, Seydoux J, Kunstner P, Aubert JF, Grouzmann E, Beermann F, et al. Cardiovascular response, feeding behavior and locomotor activity in mice lacking the NPY Y1 receptor. Nat Med. 1998;4(6):722–6. https://doi.org/10.1038/nm0698-722.
Powell AG, Apovian CM, Aronne LJ. New drug targets for the treatment of obesity. Clin Pharmacol Ther. 2011;90(1):40–51. https://doi.org/10.1038/clpt.2011.82.
Morvan F, Rondeau JM, Zou C, Minetti G, Scheufler C, Scharenberg M, et al. Blockade of activin type II receptors with a dual anti-ActRIIA/IIB antibody is critical to promote maximal skeletal muscle hypertrophy. Proc Natl Acad Sci U S A. 2017;114(47):12448–53. https://doi.org/10.1073/pnas.1707925114.
Chen JL, Walton KL, Hagg A, Colgan TD, Johnson K, Qian H, et al. Specific targeting of TGF-beta family ligands demonstrates distinct roles in the regulation of muscle mass in health and disease. Proc Natl Acad Sci U S A. 2017;114(26):E5266–E75. https://doi.org/10.1073/pnas.1620013114.
Hashimoto O, Funaba M. Activin in glucose metabolism. Vitam Horm. 2011;85:217–34. https://doi.org/10.1016/B978-0-12-385961-7.00011-1.
Lach-Trifilieff E, Minetti GC, Sheppard K, Ibebunjo C, Feige JN, Hartmann S, et al. An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy. Mol Cell Biol. 2014;34(4):606–18. https://doi.org/10.1128/MCB.01307-13.
Rooks DS, Laurent D, Praestgaard J, Rasmussen S, Bartlett M, Tanko LB. Effect of bimagrumab on thigh muscle volume and composition in men with casting-induced atrophy. J Cachexia Sarcopenia Muscle. 2017;8(5):727–34. https://doi.org/10.1002/jcsm.12205.
Hanna MG, Badrising UA, Benveniste O, Lloyd TE, Needham M, Chinoy H, et al. Safety and efficacy of intravenous bimagrumab in inclusion body myositis (RESILIENT): a randomised, double-blind, placebo-controlled phase 2b trial. Lancet Neurol. 2019;18(9):834–44. https://doi.org/10.1016/S1474-4422(19)30200-5.
Polkey MI, Praestgaard J, Berwick A, Franssen FME, Singh D, Steiner MC, et al. Activin type II receptor blockade for treatment of muscle depletion in chronic obstructive pulmonary disease. A randomized trial. Am J Respir Crit Care Med. 2019;199(3):313–20. https://doi.org/10.1164/rccm.201802-0286OC.
Garito T, Roubenoff R, Hompesch M, Morrow L, Gomez K, Rooks D, et al. Bimagrumab improves body composition and insulin sensitivity in insulin-resistant individuals. Diabetes Obes Metab. 2018;20(1):94–102. https://doi.org/10.1111/dom.13042.
Busko M. Investigational drug reduces body fat in obese patients with diabetes. In: https://www.medscape.com/viewarticle/921019. Accessed 10 Dec 2019.
Dehvari N, da Silva Junior ED, Bengtsson T, Hutchinson DS. Mirabegron: potential off target effects and uses beyond the bladder. Br J Pharmacol. 2018;175(21):4072–82. https://doi.org/10.1111/bph.14121.
Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elia E, Kessler SH, Kahn PA, et al. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab. 2015;21(1):33–8. https://doi.org/10.1016/j.cmet.2014.12.009.
Siljee JE, Unmehopa UA, Kalsbeek A, Swaab DF, Fliers E, Alkemade A. Melanocortin 4 receptor distribution in the human hypothalamus. Eur J Endocrinol. 2013;168(3):361–9. https://doi.org/10.1530/EJE-12-0750.
Yang Y. Structure, function and regulation of the melanocortin receptors. Eur J Pharmacol. 2011;660(1):125–30. https://doi.org/10.1016/j.ejphar.2010.12.020.
Huvenne H, Dubern B, Clement K, Poitou C. Rare genetic forms of obesity: clinical approach and current treatments in 2016. Obesity Facts. 2016;9(3):158–73. https://doi.org/10.1159/000445061.
Ayers KL, Glicksberg BS, Garfield AS, Longerich S, White JA, Yang P, et al. Melanocortin 4 receptor pathway dysfunction in obesity: patient stratification aimed at MC4R agonist treatment. J Clin Endocrinol Metab. 2018;103(7):2601–12. https://doi.org/10.1210/jc.2018-00258.
Kuhnen P, Clement K, Wiegand S, Blankenstein O, Gottesdiener K, Martini LL, et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N Engl J Med. 2016;375(3):240–6. https://doi.org/10.1056/NEJMoa1512693.
Clement K, Biebermann H, Farooqi IS, Van der Ploeg L, Wolters B, Poitou C et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat Med 2018;24(5):551–5. doi:https://doi.org/10.1038/s41591-018-0015-9.
Collet TH, Dubern B, Mokrosinski J, Connors H, Keogh JM, Mendes de Oliveira E, et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (setmelanotide) in MC4R deficiency. Mol Metab. 2017;6(10):1321–9. https://doi.org/10.1016/j.molmet.2017.06.015.
Chen KY, Muniyappa R, Abel BS, Mullins KP, Staker P, Brychta RJ, et al. RM-493, a melanocortin-4 receptor (MC4R) agonist, increases resting energy expenditure in obese individuals. J Clin Endocrinol Metab. 2015;100(4):1639–45. https://doi.org/10.1210/jc.2014-4024.
Tentolouris A, Vlachakis P, Tzeravini E, Eleftheriadou I, Tentolouris N. SGLT2 inhibitors: a review of their antidiabetic and cardioprotective effects. Int J Environ Res Public Health. 2019;16(16). https://doi.org/10.3390/ijerph16162965.
Shyangdan DS, Uthman OA, Waugh N. SGLT-2 receptor inhibitors for treating patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. BMJ Open. 2016;6(2):e009417. https://doi.org/10.1136/bmjopen-2015-009417.
Bays HE, Weinstein R, Law G, Canovatchel W. Canagliflozin: effects in overweight and obese subjects without diabetes mellitus. Obesity. 2014;22(4):1042–9. https://doi.org/10.1002/oby.20663.
Rajeev SP, Cuthbertson DJ, Wilding JP. Energy balance and metabolic changes with sodium-glucose co-transporter 2 inhibition. Diabetes Obes Metab. 2016;18(2):125–34. https://doi.org/10.1111/dom.12578.
Devenny JJ, Godonis HE, Harvey SJ, Rooney S, Cullen MJ, Pelleymounter MA. Weight loss induced by chronic dapagliflozin treatment is attenuated by compensatory hyperphagia in diet-induced obese (DIO) rats. Obesity. 2012;20(8):1645–52. https://doi.org/10.1038/oby.2012.59.
Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1730–5. https://doi.org/10.2337/dc15-0355.
Hollander P, Bays HE, Rosenstock J, Frustaci ME, Fung A, Vercruysse F, et al. Coadministration of canagliflozin and phentermine for weight management in overweight and obese individuals without diabetes: a randomized clinical trial. Diabetes Care. 2017;40(5):632–9. https://doi.org/10.2337/dc16-2427.
Lundkvist P, Sjostrom CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once-daily and exenatide once-weekly dual therapy: a 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab. 2017;19(1):49–60. https://doi.org/10.1111/dom.12779.
Axel AM, Mikkelsen JD, Hansen HH. Tesofensine, a novel triple monoamine reuptake inhibitor, induces appetite suppression by indirect stimulation of alpha1 adrenoceptor and dopamine D1 receptor pathways in the diet-induced obese rat. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2010;35(7):1464–76. https://doi.org/10.1038/npp.2010.16.
Hansen HH, Hansen G, Tang-Christensen M, Larsen PJ, Axel AM, Raben A, et al. The novel triple monoamine reuptake inhibitor tesofensine induces sustained weight loss and improves glycemic control in the diet-induced obese rat: comparison to sibutramine and rimonabant. Eur J Pharmacol. 2010;636(1–3):88–95. https://doi.org/10.1016/j.ejphar.2010.03.026.
Sjodin A, Gasteyger C, Nielsen AL, Raben A, Mikkelsen JD, Jensen JK, et al. The effect of the triple monoamine reuptake inhibitor tesofensine on energy metabolism and appetite in overweight and moderately obese men. Int J Obes. 2010;34(11):1634–43. https://doi.org/10.1038/ijo.2010.87.
Astrup A, Madsbad S, Breum L, Jensen TJ, Kroustrup JP, Larsen TM. Effect of tesofensine on bodyweight loss, body composition, and quality of life in obese patients: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9653):1906–13. https://doi.org/10.1016/S0140-6736(08)61525-1.
Bentzen BH, Grunnet M, Hyveled-Nielsen L, Sundgreen C, Lassen JB, Hansen HH. Anti-hypertensive treatment preserves appetite suppression while preventing cardiovascular adverse effects of tesofensine in rats. Obesity. 2013;21(5):985–92. https://doi.org/10.1002/oby.20122.
Koch M. Cannabinoid receptor signaling in central regulation of feeding behavior: a mini-review. Front Neurosci. 2017;11:293. https://doi.org/10.3389/fnins.2017.00293.
Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136(4):550–7. https://doi.org/10.1038/sj.bjp.0704767.
Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol. 2001;134(6):1151–4. https://doi.org/10.1038/sj.bjp.0704379.
Kirkham TC, Williams CM. Endocannabinoid receptor antagonists: potential for obesity treatment. Treat Endocrinol. 2004;3(6):345–60. https://doi.org/10.2165/00024677-200403060-00003.
Nagappan A, Shin J, Jung MH. Role of cannabinoid receptor type 1 in insulin resistance and its biological implications. Int J Mol Sci. 2019;20(9). https://doi.org/10.3390/ijms20092109.
Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370(9600):1706–13. https://doi.org/10.1016/S0140-6736(07)61721-8.
Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S, Group RI-ES. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365(9468):1389–97. https://doi.org/10.1016/S0140-6736(05)66374-X.
Hollander PA, Amod A, Litwak LE, Chaudhari U, Group AS. Effect of rimonabant on glycemic control in insulin-treated type 2 diabetes: the ARPEGGIO trial. Diabetes Care. 2010;33(3):605–7. https://doi.org/10.2337/dc09-0455.
Van Gaal L, Pi-Sunyer X, Despres JP, McCarthy C, Scheen A. Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care. 2008;31(Suppl 2):S229–40. https://doi.org/10.2337/dc08-s258.
Mitschke MM, Hoffmann LS, Gnad T, Scholz D, Kruithoff K, Mayer P, et al. Increased cGMP promotes healthy expansion and browning of white adipose tissue. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 2013;27(4):1621–30. https://doi.org/10.1096/fj.12-221580.
Li S, Li Y, Xiang L, Dong J, Liu M, Xiang G. Sildenafil induces browning of subcutaneous white adipose tissue in overweight adults. Metab Clin Exp. 2018;78:106–17. https://doi.org/10.1016/j.metabol.2017.09.008.
Carbone F, Tack J. The effect of sildenafil on gastric motility and satiation in healthy controls. United European Gastroenterol J. 2018;6(6):846–54. https://doi.org/10.1177/2050640618766933.
Liang C, Curry BJ, Brown PL, Zemel MB. Leucine modulates mitochondrial biogenesis and SIRT1-AMPK signaling in C2C12 myotubes. Journal of Nutrition and Metabolism. 2014;2014:239750. https://doi.org/10.1155/2014/239750.
Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, et al. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443(3):285–9. https://doi.org/10.1016/s0014-5793(98)01705-0.
Fu L, Li F, Bruckbauer A, Cao Q, Cui X, Wu R, et al. Interaction between leucine and phosphodiesterase 5 inhibition in modulating insulin sensitivity and lipid metabolism. Diabetes, Metabolic Syndrome and Obesity : Targets and Therapy. 2015;8:227–39. https://doi.org/10.2147/DMSO.S82338.
Zemel MB, Kolterman O, Rinella M, Vuppalanchi R, Flores O, Barritt AS, et al. Randomized controlled trial of a leucine-metformin-sildenafil combination (NS-0200) on weight and metabolic parameters. Obesity. 2019;27(1):59–67. https://doi.org/10.1002/oby.22346.
Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr. 2008;88(4):906–12. https://doi.org/10.1093/ajcn/88.4.906.
Ostendorf DM, Caldwell AE, Creasy SA, Pan Z, Lyden K, Bergouignan A, et al. Physical activity energy expenditure and total daily energy expenditure in successful weight loss maintainers. Obesity. 2019;27(3):496–504. https://doi.org/10.1002/oby.22373.
Melby CL, Paris HL, Sayer RD, Bell C, Hill JO. Increasing energy flux to maintain diet-induced weight loss. Nutrients. 2019;11(10). https://doi.org/10.3390/nu11102533.
Petridou A, Siopi A, Mougios V. Exercise in the management of obesity. Metab Clin Exp. 2019;92:163–9. https://doi.org/10.1016/j.metabol.2018.10.009.
Foright RM, Presby DM, Sherk VD, Kahn D, Checkley LA, Giles ED, et al. Is regular exercise an effective strategy for weight loss maintenance? Physiol Behav. 2018;188:86–93. https://doi.org/10.1016/j.physbeh.2018.01.025.
Zinman B, Bhosekar V, Busch R, Holst I, Ludvik B, Thielke D, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356–67. https://doi.org/10.1016/S2213-8587(19)30066-X.
Frias JP, Guja C, Hardy E, Ahmed A, Dong F, Ohman P, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(12):1004–16. https://doi.org/10.1016/S2213-8587(16)30267-4.
Ludvik B, Frias JP, Tinahones FJ, Wainstein J, Jiang H, Robertson KE, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(5):370–81. https://doi.org/10.1016/S2213-8587(18)30023-8.
Miras AD, Perez-Pevida B, Aldhwayan M, Kamocka A, McGlone ER, Al-Najim W, et al. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(7):549–59. https://doi.org/10.1016/S2213-8587(19)30157-3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Obesity Treatment
Rights and permissions
About this article
Cite this article
Tsilingiris, D., Liatis, S., Dalamaga, M. et al. The Fight Against Obesity Escalates: New Drugs on the Horizon and Metabolic Implications. Curr Obes Rep 9, 136–149 (2020). https://doi.org/10.1007/s13679-020-00378-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-020-00378-x