Abstract
Purpose of Review
The coronavirus disease 2019 (COVID-19) pandemic has challenged global health systems and economies from January 2020. COVID-19 caused by the infectious severe acute respiratory syndrome coronavirus (SARS-CoV-2) has acute respiratory and cardiometabolic symptoms that can be severe and lethal. Long-term physiological and psychological symptoms, known as long COVID-19, persist affecting multiple organ systems. While vaccinations support the fight against SARS-CoV-2, other effective mechanisms of population protection should exist given the presence of yet unvaccinated and at-risk vulnerable groups, global disease comorbidities, and short-lived vaccine responses. The review proposes vitamin D3 as a plausible molecule for prevention, protection, and disease mitigation of acute and long COVID-19.
Recent Findings
Epidemiological studies have shown that individuals who were deficient in vitamin D3 had worse COVID-19 health outcomes and mortality rates. Higher doses of vitamin D3 supplementation may improve health and survivorship in individuals of various age groups, comorbidities, and severity of disease symptoms.
Summary
Vitamin D3’s biological effects can provide protection and repair in multiple organ systems affected by SARS-CoV-2. Vitamin D3 supplementation can potentially support disease-mitigation in acute and long COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The COVID-19 Pandemic in Global Perspective
The coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus (SARS-CoV-2) which was declared a Public Health Emergency of International Concern by the World Health Organization (WHO) on 30 January 2020. To date, 29 March 2023, according to WHO, 761,402,282 cases of SARS-CoV-2 infection have been confirmed, and 6,887,000 deaths have been reported due to COVID-19 [1]. Lockdowns, boarder closures affecting trade and travel, morbidity and mortality associated with COVID-19 have been globally devastating. The International Monetary Fund reported that the global economy contracted by 3.5% in 2020 alone [2], and 90% of the global economy experienced a contraction in per capita GDP [3]. While the emotional value of losing a loved one is immeasurable, the economic cost using the value per statistical life (VSL) estimate is $10 million per lost life [4]. Long-term economic losses remain elusive. While global economic recovery is predicted, it is even more important to ensure recovery of population health and well-being. Such recovery needs to promote lifestyle behaviors that would mitigate future risk and burden from newly emergent microbes and pandemics of similar public health scale. This continues to be pertinent because new SARS-CoV-2 variants continue to emerge, and global countries, including China, continue to enforce lockdowns to prevent COVID-19 outbreaks when needed. Moreover, recent findings indicate that COVID-19 has novel long-term physiological and psychological effects that affect multiple organ systems with observed neurological, inflammatory, metabolic, muscular, reproductive, cardiac, and psychological symptoms, among others, which can persist. Such novel manifestations may occur in SARS-CoV-2-infected individuals regardless of initial disease severity or symptoms. Vaccinations have minimized global infection rates of SARS-CoV-2 and COVID-19 disease burden, but are inequitably available globally, have short-lived efficacy and recently reported side effects. Therefore, other effective mechanisms of population protection should exist especially in the presence of yet unvaccinated, underprivileged, and at-risk groups, and short-lived vaccine responses that require constant boosters or have side effects that cause concern. The need is pertinent in the presence of highly prevalent metabolic disease comorbidities in global populations, such as cardiovascular disease and obesity, and continuous emergence of new variants. Additionally, other previously dormant microbes, e.g., monkeypox virus, Marburg virus, and Crimean-Congo hemorrhagic fever virus have reemerged recently with greater virulence, reminding everyone that potential similar public health dangers are always lurking. Considering the above, and the enigmatic emergent long COVID-19 symptoms, it becomes a public health need to present sustainable, accessible, cost-effective health solutions for disease prevention and management.
SARS-CoV-2, Coronaviruses, and Infectivity
SARS-CoV-2 is a ribovirus member of the Coronaviridae family of viruses. Coronaviruses are zoonotic and can infect several species including bats, cats, rodents, dogs, pigs, birds, cows, minks, camels, rabbits, and humans, all of which are viral reservoirs of transmission [5, 6]. SARS-CoV-2 is the 7th coronavirus that is known to infect humans. The SARS-CoV-2 virus shares 79% genetic homology with SARS-CoV-1 (2002 pandemic), 50% homology with Middle East respiratory syndrome coronavirus (MERS-Co-V) (2012 pandemic) and high sequence homology with several other Rhinolophus coronaviruses isolated in bats but which have yet not bridged from animal reservoirs to infect humans [7].
SARS-CoV-2 is a positive-sense single-stranded RNA-virus. It has six functional open reading frames (ORFs) coding for essential structural proteins that promote viral replication and infectivity: replicase, spike (S), envelop, membrane and nucleocapsid proteins. Additionally, seven other ORFs encode accessory proteins [8]. Viral infection involves the spike viral protein subunits S1 and S2 and the Angiotensin Converting Enzyme 2 (ACE2) receptors present in human host tissues [9]. Several variants of SARS-CoV-2 have been reported especially those mutated at the S domain, with six major variants of concern reported to be more infective by WHO [10].
SARS-CoV-2 Pathophysiology in the Short and Long Term
Infection with SARS-CoV-2 is problematic in the short and long term. In the short term, viral infection and ensuing ‘cytokine storm’ may result in mild to severe physiological respiratory, hematological, renal, cardiac, and gastrointestinal symptoms that may cause morbidities and mortality [11•]. Post infection, over 55 residual long COVID-19 symptoms may develop or persist which affect multiple organ systems with emergent novel complications. These can include immunological, metabolic, musculoskeletal and nervous system disorders such as weakness, fatigue, dyspnea, sarcopenia, incontinence, neuropathies, encephalopathies, cerebral strokes [12, 13], increase in endocrine disturbances such as new-onset diabetes mellitus [14], hair loss, anosmia, dysgeusia, headache, attention disorder, deteriorating brain and cognition functions, declining mental health, among other complications [15, 16, 17•]. Often these symptoms of long COVID-19 persist up to and over 60 days after disease onset [18]. Increasing age, female sex, white ethnicity, poor pre-pandemic general and mental health including metabolic comorbidities, immunocompromization, obesity, and asthma, are associated with prolonged symptoms [17•, 19, 20, 21•]. Given that the SARS-CoV-2 virus has the potential to invade ACE2 receptor-containing cells and tissues, endothelial cells on vessels and organs, leukocytes, and dendritic cells, this implies that potentially there are many effects to the virus that are yet unknown and could cause multi-organ system long-term dysfunction. Some of these organs and tissues include kidneys, adipose tissues, nerves, heart, pancreas, testes, among others [22]. Given SARS-CoV-2’s versatility in infecting multiple organ system targets, scientists need to find biological molecules with similar omnipotent diversity of action that could protect multiple organs and mitigate disease burden. These molecules’ actions would include priming individuals’ immunity, reducing viral replication and infection, attenuating comorbidities’ risk, promoting organ repair and recuperation, enhancing baseline health, and promoting survival. We propose that vitamin D3 could be a cost-effective biological molecule that exerts such diverse biological effects on multiple tissues and systems [23], and promotes protective and regenerative mechanisms against SARS-CoV-2, acute and long COVID-19.
Vitamin D3 has Many Health Benefits for Multiple Organ Systems and Well-being
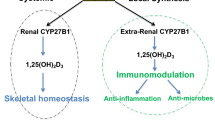
Vitamin D3 can be synthesized in the human body following sun exposure. Synthesis begins via the skin by conversion of 7-dehydrocholesterol to vitamin D3 (also termed cholecalciferol). A 15-min half body exposure produces approximately 10,000 International Units (IU) (250 mcg) to 20,000 IU (500 mcg) of vitamin D3, cholecalciferol. This depends on several factors including duration of sun exposure and skin color. Upon sun avoidance or low sun exposure due to different ecological latitudes, vitamin D3 may need to be ingested from foods such as oily fish, eggs, fortified juice or milk or cereals, and a range of animal-based products such as liver. Vitamin D3 synthesized via the skin, is then metabolized in the liver to 25-hydroxyvitamin D3 (25(OH)D3) (termed calcidiol), followed by conversion in the kidneys to the active biological form 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) (termed calcitriol). Regular daily sun exposure or supplementation can support reaching physiological vitamin D3 sufficiency which is attained with a serum 25(OH)D3 concentration at or greater than 30 ng/mL [24•]. The National Academy of Sciences, Engineering and Medicine (NASEM) (previously Institutes of Medicine (IOM)) recommends a somewhat conservative daily recommended dietary allowance (RDA) for vitamin D3 of 600 IU/day (15 mcg/day). The Endocrine Society recommends at least 1500–2000 IU/day (37.5–50 mcg/day) and up to 4,000 IU/day (100 mcg/day) for adults, with 10,000 IU/day (250 mcg/day) as upper tolerable level (UL). Deficiencies can be treated with supplementation of up to 50,000 IU/week (1,250 mcg/week). The above recommendations require revision as studies have revealed a requirement for significantly higher vitamin D3 concentrations for health maintenance and disease prevention, and adjustment of RDAs and ULs according to individual baseline vitamin D3 concentrations [25•, 26, 27].
Many in vitro, animal model, translational, observational, and clinical studies have confirmed a variety of roles for vitamin D3 in maintaining health and preventing disease. Vitamin D3, an important nutrient and hormone exerts its biological effects via binding its vitamin D receptor (VDR) and modulating downstream gene transcription of hundreds of genes in multiple organ systems. Some of vitamin D3’s roles include improvement in brain functions such as cognition, memory and mood, supporting calcium homeostasis and bone remodeling, enhancing cardiometabolic health and endothelial function, regulating blood pressure, improving insulin secretion [28•] and sensitivity, and supporting placental function in pregnant women [23].
Vitamin D3 can modulate both the innate and adaptive immune system [29]. At airway epithelia, vitamin D3 enhances secretion of antimicrobial peptides such as β-defensins and cathelicidins which inhibit the cellular entry and subsequent proliferation of virus particles. Vitamin D3 induces upregulation of cathelicidin human cationic antimicrobial protein (hCAP18), in neutrophils, natural killer cells, monocytes, and B-cells. In macrophages, vitamin D3 enhances autophagy through upregulation of calcium and nitric oxide levels, inhibition of mammalian target of rapamycin (mTOR) thus promoting clearance of virus-infected cells. Vitamin D3 could therefore potentially override the SARS-CoV-2 ORF3a impairment of autophagy, and its immune-modulating properties in response to SARS-CoV-2 infection should be further studied.
Vitamin D3 is an effective antioxidant and anti-inflammatory agent. Vitamin D3 sufficiency significantly protects against diseases where inflammation is a hallmark of disease progression. These include cancer, Alzheimer’s disease, Multiple Sclerosis, and rheumatoid arthritis [29]. Vitamin D3 can combat the anti-inflammatory ‘cytokine storm’ and fibrotic effects associated with COVID-19. Vitamin D3 upregulates the expression of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha (IkBα) which inhibits the proinflammatory transcription factor nuclear factor kappa light-chain enhancer of activated B cells (NFkB), resulting in the reduced expression of inflammatory genes. In dose and cell-specific mechanisms, vitamin D3 can modulate gene expression of Interleukins IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, and IL-15, Interferon gamma (IFN-γ), Tumour growth factor beta (TGF-β) and Tumor necrosis factor alpha (TNF-α) in directions that promote anti-inflammatory and tissue repair pathways [30, 31].
In the adaptive immune system, the VDR receptor is expressed in activated mature T-cells. Immunomodulatory effect of 1,25(OH)2D3 on CD4+ T-cells involves suppression of the proinflammatory Type-1 T helper (Th1) and Type-17 T-helper (Th17) subsets that cause tissue damage, in favor of activation of the Type-2 T helper (Th2) cells and Induced regulatory T-cells (iTreg). This shift towards Th2 and iTreg subsets favors B-cell activation in humoral immunity, anti-inflammatory effects, and tissue healing pathways through dose-dependent TGF-β induction. Studies do not show that vitamin D3 promotes T-cell proliferation from naive precursors [32•].
In studies on RNA stranded viruses such as Respiratory Syncytial Virus, H9N2 Influenza virus, rotavirus, Human Immunodeficiency Virus, vitamin D3 demonstrated antiviral activity, reduction of viral load, select modulation of viral gene transcription, or protection of cells against virus-induced cell death. However, vitamin D3 effects on different classes of DNA and RNA viruses remain to be examined in big clinical trials as its effects are currently contradictory depending on the virus types and severity of infection [30, 33]. Vitamin D3 needs to be studied for its potential anti-viral effects against SARS-CoV-2.
Vitamin D3 Supplementation Mitigates COVID-19 Disease Severity, Morbidity and Improves Survival in Different Age Groups
Several epidemiological studies have explored the link between vitamin D3 and SARS-CoV-2 or COVID-19. While heterogenous in type, criteria, and results, collectively these studies showed that vitamin D3 deficiency or insufficiency were associated with increased risk of SARS-CoV-2 infection and COVID-19 severity [34]. In a matched retrospective case-control study on 464,383 participants in two matched case-control groups of individuals for which 25(OH)D3 concentrations and body mass index (BMI) were available before the pandemic, results showed that individuals with very low 25(OH)D3 concentrations (< 30 nmol/L) had the highest risks for SARS-CoV-2 infection, and for COVID-19 severity when infected; Odds Ratio (OR) were 1.246 [95% Confidence Interval (CI): 1.210–1.304] and 1.513 [95% CI: 1.230–1.861], respectively [35]. In a retrospective observational study of 191,779 participants in the USA, Kaufman et al. showed that SARS-CoV-2 infection incidence was higher in individuals with lower serum 25(OH)D3 concentrations. The SARS-CoV-2 positivity rate was higher in the 39,190 individuals with 25(OH)D3 deficiency (< 20 ng/mL) [12.5%, 95% C.I. 12.2–12.8%] than in the 27,870 individuals with sufficient serum 25(OH)D3 concentrations (30–34 ng/mL) [8.1%, 95% C.I. 7.8–8.4%] and in the 12,321 individuals with serum 25(OH)D3 ≥ 55 ng/mL [5.9%, 95% C.I. 5.5–6.4%]. This association held true for individuals across geographical latitudes, ethnicities, sexes and age groups [36].
In a study of 70-year-old elderly individuals hospitalized for COVID-19, Sulli et al. showed that 25(OH)D3 serum deficiency was associated with more severe lung involvement, longer disease duration, and risk of death. In these elderly participants, 25(OH)D3 levels were significantly lower compared to controls, (median 7.9 vs. 16.3 ng/mL, respectively, p = 0.001). Lower 25(OH)D3 serum concentrations were significantly correlated with elevated D-Dimer and C-reactive proteins concentrations, two important indicators of coagulopathy and inflammation. A negative correlation was observed between 25(OH)D3 serum concentrations and severity of radiologic pulmonary involvement and mortality during hospitalization. Higher 25(OH)D3 serum concentrations were associated with improved pulmonary parameters such as partial oxygen pressure (PaO2) (p = 0.03), oxygen saturation (sO2) (p = 0.05), and the ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen (PaO2/FiO2) (p = 0.02). Elderly individuals with low 25(OH)D3 had multiple lung consolidations with diffuse severe interstitial lung involvement compared to those with higher 25(OH)D3 concentrations who had milder lung symptoms. These studies above and other similar investigations suggest that maintaining higher Vitamin D3 status prior to infection could be protective and reduce the severity of COVID-19-induced organ injury [37].
The timing of vitamin D3 administration may also be associated with improved health outcomes. A quasi-experimental study in Italy compared the effects of vitamin D3 cholecalciferol administration on improving survival outcomes in elderly hospitalized individuals. Participants were divided into three groups, Group 1 administered vitamin D3 for the year preceding infection and hospitalization (bolus included the doses of 50,000 IU vitamin D3 per month, or the doses of 80,000 IU or 100,000 IU vitamin D3 every 2–3 months), Group 2 administered an oral supplement of 80,000 IU vitamin D3 within a few hours of the diagnosis of COVID-19, and a third no-supplementation reference Group 3. Survival of participants were, in Group 1 (93.1%), Group 2 (81.2%) and Group 3 (68.7%) respectively, (p = 0.02 between groups 1 and 3). After correction for any confounding variables, using Group 3 as reference hazard ratio (HR) = 1, the adjusted HR for 14-day mortality was HR = 0.07 (p = 0.017) for Group 1 and HR = 0.37 (p = 0.28) for Group 2. Group 1 had longer survival time than Group 3 (log-rank p = 0.015), although there was no survival difference between Groups 2 and 3 (log-rank p = 0.32). Group 1, but not Group 2 (p = 0.40), was associated with improved clinical outcomes using an ordinal scale for clinical improvement (OSCI). These findings show that regular bolus vitamin D3 supplementation as a preventive habit or lifestyle before viral infection was associated with better outcome and survival in frail elderly individuals [38, 39]. This finding consolidates data from other studies which show that individuals who have sufficient concentrations of 25(OH)D3 in their blood stream due to previous adequate vitamin D3 intake or sun exposure have a much higher survival rate that those who are deficient in vitamin D3. This implies that maintaining vitamin D3 sufficiency in individuals before infection can have preventive value and public health benefits. Sustained supplementation with vitamin D3 should be considered, and further population studies should be performed to verify supplementation needs, doses, and regimen.
A randomized open-label, double-masked clinical trial examined the effect of administering cholecalciferol to hospitalized individuals in addition to the best available therapy present at the time, namely hydroxychloroquine or azithromycin. Upon randomization in ratio 2:1 cholecalciferol to no-cholecalciferol respectively, cholecalciferol was given orally as 0.532 mg (21,280 IU) upon admission, then 0.266 mg (10,640 IU) on day 3 and 7, and then weekly until discharge or Intensive Care Unit (ICU) admission. From the 50 participants on cholecalciferol treatment, only 1 required ICU admission (2%), none died, and all were discharged without major complications. In the 26 control/no-cholecalciferol treatment participants, 13 (50%) required ICU admissions, two of whom died. When adjusting for comorbidities, hypertension and type 2 diabetes mellitus, the OR for ICU admission in the cholecalciferol treatment versus controls was OR = 0.03 [95% CI: 0.003–0.25]. None of the treated participants suffered any side effects from the higher dose cholecalciferol [40].
In contrast, Abroug et al. showed in a randomized controlled trial (RCT) that if a single vitamin D3 treatment of 200,000 IU was given to individuals with moderate to severe SARS-CoV-2 infection but after 14 days after initial diagnosis, such a treatment does not appear to have a significant effect on shortened recovery time in vitamin D-treated individuals compared to participants given a placebo [41].
In a double-blind RCT conducted in Brazil, Murai et al. investigated the effects of a single oral dose of treatment with 200,000 IU of vitamin D3 versus placebo on the clinical outcomes of 240 individuals hospitalized with moderate to severe COVID-19 symptoms. Analysis of the RCT data showed that there were no significant differences between individuals in the vitamin D3 treated group versus the placebo group in the need for mechanical ventilation (7.6% versus 14.4%; difference, -6.8% [95% CI, -15.1% to 1.2%]; p = 0.09), admission to ICU (16.0% versus 21.2%; difference, -5.2% [95% CI, -15.1% to 4.7%]; p = 0.30), mean length of hospital stay (7.0 [4.0–10.0] days versus 7.0 [5.0–13.0] days; log-rank p = 0.59), and in-hospital mortality (7.6% versus 5.1%; difference, 2.5% [95% CI, -4.1% to 9.2%]; p = 0.43). The high dose treatment successfully elevated serum 25(OH)D3 concentrations with no noteworthy adverse events reported. The findings may imply that treatment with vitamin D3 after the onset of moderate to severe short COVID-19 cascade of symptoms may not be so effective at improving clinical outcomes and survival [42].
In a prospective observational study conducted on 410 subjects affected by COVID-19 in India, Jevalikar et al. found no association between baseline 25(OH)D3 and severity of COVID-10 symptoms or survival rates. The investigators compared two groups of participants; Group 1 had participants who had vitamin D3 deficiency (VDD) with 25(OH)D3 < 20 ng/ml versus Group 2 which included participants who did were not classified as vitamin D3 deficient. Jevalikar et al. showed that the proportion of severe cases (13.2% vs.14.6%), mortality (2% vs. 5.2%), oxygen requirement (34.5% vs.43.4%), and ICU admission (14.7% vs.19.8%) was not significantly different between individuals with or without VDD, respectively. A limitation of this study lies in the fact that participants with VDD were significantly younger and had lesser comorbidities, which may have influenced some of the outcomes and findings [43], in contrast to several other studies that reported an association with vitamin D deficiency and disease severity [44•].
In a multicenter non-randomized cohort study, 537 participants who were hospitalized in 5 hospitals in Spain were administration capsules of the active metabolite calcifediol, 25(OH)D3 upon entry and throughout a 30-day hospitalization period. The dose per capsule was 0.266 mg/capsule and administered as 2 capsules on entry and then one capsule on day 3, 7, 14, 21, and 28. Participants receiving 25(OH)D3 had lower comorbidity and score of pneumatic severity (including blood pressure measures, urea, respiratory distress), lower C-reactive protein indicative of lower inflammation, lower rates of chronic kidney disease and blood urea nitrogen. In the group that received the 25(OH)D3, mortality rate was 5% compared to 20% in the no-treatment group with OR = 0.22 in these 25(OH)D3 recipients compared to controls [95% CI, 0.08 to 0.61] [45].
The COvid19 and VITamin D TRIAL (COVIT-TRIAL), a multicentered, open-label RCT conducted in France tested whether a single high oral dose of vitamin D3 cholecalciferol of 400,000 IU administered within 72 h after SARS-CoV-2 infection could improve overall 14-day survival of elderly participants compared to standard-dose, 50,000 IU participants. Fourteen days following infection, participants allocated to higher dose cholecalciferol suffered fewer deaths (6%) compared to participants on lower dose (11%), adjusted HR = 0.39 [95% CI: 0.16 to 0.99] (p = 0.045). This protective effect was not sustained for 28-day survival. Higher dose cholecalciferol did not result in more frequent adverse effects compared to the standard dose [38, 46].
In a study on 78 healthcare workers infected by SARS-CoV-2, supplementation of vitamin D3 resulted in milder symptoms and clinical features. Two groups were treated for three months. Group 1 participants received supplementation of 50,000 IU/week for two weeks followed by daily doses of 5,000 IU/day, while participants in Group 2 were supplemented with 2,000 IU/day for three months. Normalization of serum 25(OH)D3 concentrations was achieved in 53% of those on the higher dose of vitamin D3 supplementation. Vitamin D3 serum concentrations could not be correlated in this small size study with reduced morbidity. However, data showed that participants receiving higher dose of 5,000 IU/day vitamin D3 supplementation had milder to no symptoms of SARS-CoV-2 compared to participants on lower 2,000 IU/day suggesting potential protective effects against severity of symptoms of COVID-19. Neither vitamin D3 intake nor vitamin D3 deficiency or insufficiency were associated with a decrease in SARS-CoV-2 - associated morbidity [47]. The above studies collectively suggest that supplementation with higher doses of vitamin D3 could support reduction in COVID-19 disease severity and symptoms, and a moderate improvement in survival, by promoting earlier and faster recovery mechanisms. The timing of vitamin D3 administration appears to be important. Several studies suggest that maintaining vitamin D3 sufficiency before infection or disease symptoms, or supplementing with vitamin D3 at early stages of disease progression may achieve significantly reduced symptoms and improved clinical outcomes in individuals with COVID-19 [44•, 48•].
Vitamin D3 Could Improve Brain Function in Individuals Affected by Long COVID-19
COVID-19 can cause physiological and cognitive abnormalities in acute and long COVID-19 [49]. The acute underlying causes of brain dysfunction due to the SARS-CoV-2 may involve inflammatory damage in the brain both in the neurons and the endothelial vasculature, instigated by viral infiltration and endothelial cell activation. Leaky endothelium would enable complement and immune cell infiltration of the protective blood brain barrier [50]. In addition to the ensuing hypercoagulation or hyperinflammation, the virus interferes with neurological functions which may explain the brain’s long COVID-19 symptoms of fatigue, brain fog, loss of focus, memory deficit, and other cognitive abnormalities. Vitamin D3 potentially could abrogate these symptoms, protect brain function, and promote reparative healing even after infection. Both 25(OH)D3 and 1,25(OH)2D3 can cross the blood brain barrier. In stroke patients, vitamin D3 has a protective and reparative effect on neuronal damage especially in ischemic stroke symptoms [51•]. Vitamin D3 plays an integral role in maintaining brain plasticity, enabling proficient cognitive function, and preventing neurodegeneration. Vitamin D3 deficiency is associated with developmental neuronal functional abnormalities in growing fetuses, poor memory and concentration in adults, lower cognitive function, reduced brain volume and hippocampus function in adults, as well as neurodegenerative or psychological diseases such as Alzheimer’s, Multiple Sclerosis, and depression [52,53,54]. The association with low vitamin D3 and worse cognitive performance is more pronounced in women with depression [53]. Interestingly, more women than men are affected by the brain cognitive dysfunction associated with long COVID-19. Apropos, the repletion, sufficiency, and administration of vitamin D3 to protect against the long COVID-19-associated brain abnormalities should be considered and further studied.
Vitamin D3 Could Improve Muscle Regeneration and Repair in Individuals with Long COVID-19
Many individuals experiencing long COVID-19 symptoms present with fatigue, weakness, and myalgia for reasons that remain to be elucidated. Lessons from diseases such as Chronic obstructive pulmonary disease (COPD) allude to the possibility that vitamin D3 is a feasible option to improve disease-associate muscle function. In COPD, obstructive lung disease of the peripheral airways occurs, and is associated with inflammation, hypertension, diabetes mellitus, skeletal muscle dysfunction, and osteoporosis. Obstruction and limitations of airflow in patients reduces oxygen supply, produces a decline in aerobic capacity and increases the generation of reactive oxygen species that cause oxidation, chronic inflammation, and destruction of type I muscle fibers, muscle wasting, loss of muscle strength and fatigue. In extreme cases, this can cause sarcopenia and cachexia. Vitamin D3 supplementation in such individuals ameliorates inflammation, improves lung function, promotes mitochondrial repair, hence improves muscle regeneration and rebuild over time [55]. A meta-analysis examining individuals with vitamin D3 deficiency (25(OH)D3 ≤ 25 nmol/l) showed that supplementation with different vitamin D3 doses (from 4,000 to 60,000 IU per week) significantly improved upper and lower muscular body strength. This meta-analysis examined 7 RCTs including 310 adults, 67% of whom were females, ages ranging 21.5–31.5 years [56•]. The regenerative mechanisms rely on vitamin D3’s ability to enhance mitochondrial function, increase satellite muscle stem cell recruitment, and promote myogenic repair [57]. In long COVID-19, such musculoskeletal complaints exist and are more prevalent in young female adults. This same argument for using vitamin D3 supplementation in improving mitochondrial function, myogenic repair, enhancing muscular body strength, ameliorating inflammation or reperfusion injury muscle dysfunction, and hence treating individuals with long COVID-19-associated muscle fatigue should be examined in future RCTs.
Vitamin D3 Reduces Risk in COVID-19 Individuals Who Have Metabolic Comorbidities
Vitamin D3 promotes cardiometabolic health benefits. Vitamin D3 is important in blood pressure regulation, insulin sensitivity, downregulation of inflammatory responses, and reduction of risk for cardiometabolic diseases [28•]. COVID-19 complications include blood pressure abnormalities, new-onset diabetes mellitus and COVID-19-associated β-cell dysfunction [14], abnormal coagulopathy, and hyperinflammation associated with an inflammatory ‘cytokine storm’ [11•]. Endothelial damage either by hyperinflammation, oxidative stress, or metabolic irregularities e.g., hyperleptinemia, hyperinsulinemia or hyperglycemia exacerbate this risk. Many of the at-risk COVID-19 affected individuals suffer from comorbidities cardiovascular diseases, diabetes mellitus, hypertension, and obesity [12, 20]. Knowing the beneficial effects of vitamin D3 in attenuating cardiometabolic risk factors presents Vitamin D3 as a plausible biological agent to mitigate COVID-19 disease burden.
In a study of 43 individuals infected with SARS-CoV-2, Tan et al. examined the benefits of administering a combination therapy of vitamin D3, magnesium and vitamin B12 on severe outcome progression in older individuals above the age of 50 most of whom suffered from diabetes mellitus or hypertension. The SARS-CoV-2 infected individuals were given a combination treatment made up of 1,000 IU vitamin D3, 150 mg magnesium oxide, and 500 mcg vitamin B12 daily for up to 14 days compared to controls. Individuals given the combination treatment had fewer requirements for oxygen therapy during hospitalization in ICU or on ward (17.6% vs. 61.5%, respectively, p = 0.006). Additionally, combination treatment was associated with a lower likelihood for intensive care support. In multivariate analysis after adjusting for age and hypertension risk factors, participants given the combination treatment still had a lower need for oxygen therapy and intensive care support OR = 0.20 [95% CI:0.04 -0.93; p = 0.04] compared to controls. This suggested that vitamin D3 in combination treatment can have a beneficial and protective survival effects on older at-risk individuals with COVID-19 who had pre-existing comorbidities. The combination treatment may have metabolic and immunomodulatory effects, with vitamin D3 and magnesium exerting anti-inflammatory, anti-hypertensive, anti-thrombotic and broncho-dilatory effects, while vitamin D3 and vitamin B12 supporting gut microbiome development and priming innate and adaptive immune responses. While the study’s sample size is small it does incentivize investigating such combination nutrient treatments, including vitamin D3 supplementation, in at-risk individuals with comorbidities [58].
Conclusion
Maintaining vitamin D3 sufficiency prior to infection appears to be important in reducing risk and severity of COVID-19 in individuals of all ages. Furthermore, given vitamin D3’s well-known protective and regenerative physiological effects in multiple organ systems, administration of vitamin D3 to individuals infected with SARS-CoV-2 may promote faster recovery times and improved survival. Specific vitamin D3-induced mechanisms of action in individuals suffering from short or long COVID-19 need to be clearly elucidated, and supplementation studies consolidated. Nonetheless, cumulative evidence increasingly supports a potential role for the use of vitamin D3 in mitigating acute and long COVID-19 symptoms and disease burden, and in repairing disease-associated organ damage. No side effects have been reported following higher vitamin D3 intake as seen in epidemiological studies on individuals affected by COVID-19. Apropos, vitamin D3 supplementation, study design, and dosing regimens need to be revised to include higher doses of vitamin D3 in future studies, compared to current practices [46, 59•]. This is especially pertinent in at-risk subgroups, such as the elderly and individuals with obesity, who may benefit from higher-dose supplementation for various physiological reasons [60, 61]. Vitamin D3's potential as a cost-effective candidate in the management and mitigation of COVID-19 disease burden warrants further investigation given vitamin D3's multipotent diverse mechanistic actions in maintaining health and preventing disease.
Data Availability
The papers and data supporting this review are all available as publications in public sources such as PubMed and other search engines. There is no unique empirical data generated for this review to be shared or accessed.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
WHO. WHO Coronavirus dashboard. https://covid19.who.int/. Accessed 2 Apr 2023.
Yeyati EL, Filippini F. Social and economic impact of COVID-19. In: The Independent Panel of the World Health Organization, editor. Brookings global working paper #158. Brookings Institution; 2021.
The World Bank. Subdued global economic recovery. In: Global economic prospects. Washington, DC: The World Bank; 2021.
Robinson LA. COVID-19 and uncertainties in the value per statistical life. The Regulatory Review; 2020.
Ye ZW, Yuan S, Yuen KS, Fung SY, Chan CP, Jin DY. Zoonotic origins of human coronaviruses. Int J Biol Sci. 2020;16(10):1686–97. https://doi.org/10.7150/ijbs.45472.
Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–54. https://doi.org/10.1038/s41579-020-00459-7.
Chen B, Tian EK, He B, Tian L, Han R, Wang S, et al. Overview of lethal human coronaviruses. Signal Transduct Target Ther. 2020;5(1):89. https://doi.org/10.1038/s41392-020-0190-2.
Rahimi A, Mirzazadeh A, Tavakolpour S. Genetics and genomics of SARS-CoV-2: A review of the literature with the special focus on genetic diversity and SARS-CoV-2 genome detection. Genomics. 2021;113(1 Pt 2):1221–32. https://doi.org/10.1016/j.ygeno.2020.09.059.
Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80. https://doi.org/10.1016/j.cell.2020.02.052.
WHO. Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update. World Health Organization; 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed 2 April 2023.
• Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5. This is one the first seminal descriptions of clinical features of patients with acute COVID-19 emerging from China at the beginning of the pandemic.
Silva Andrade B, Siqueira S, de Assis Soares WR, de Souza Rangel F, Santos NO, Dos Santos Freitas A, et al. Long-COVID and Post-COVID health complications: an up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses. 2021;13(4):700. https://doi.org/10.3390/v13040700.
Al-Olama M, Rashid A, Garozzo D. COVID-19-associated meningoencephalitis complicated with intracranial hemorrhage: a case report. Acta Neurochir. 2020;162(7):1495–9. https://doi.org/10.1007/s00701-020-04402-w.
Sathish T, Cao Y, Kapoor N. Newly diagnosed diabetes in COVID-19 patients. Prim Care Diabetes. 2021;15(1):194. https://doi.org/10.1016/j.pcd.2020.08.014.
Sierpina VS. Become aware of the short and long-term mental health effects of COVID. Explore. 2021;17(4):281. https://doi.org/10.1016/j.explore.2021.04.008.
Gemelli Against C-P-ACSG. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613–20. https://doi.org/10.1007/s40520-020-01616-x.
• Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. https://doi.org/10.1038/s41598-021-95565-8. This systematic review details more than 50 long COVID-19 complications reported.
Carfi A, Bernabei R, Landi F, Gemelli Against C-P-ACSG. Persistent symptoms in patients after acute COVID-19. Jama. 2020;324(6):603–5. https://doi.org/10.1001/jama.2020.12603.
Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton IJ. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehab Med. 2020;52(8):jrm00089. https://doi.org/10.2340/16501977-2727.
Garg MK, Gopalakrishnan M, Yadav P, Misra S. Endocrine involvement in COVID-19: mechanisms, clinical features, and implications for care. Indian J Endocrinol Metab. 2020;24(5):381–6. https://doi.org/10.4103/ijem.IJEM_440_20.
• Thompson EJ, Williams DM, Walker AJ, Mitchell RE, Niedzwiedz CL, Yang TC, et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat Commun. 2022;13(1):3528. https://doi.org/10.1038/s41467-022-30836-0. This paper reviews long COVID-19 disease burden and risk factors available from 10 UK longtitudinal studies.
Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. https://doi.org/10.1186/s40249-020-00662-x.
Baggerly CA, Cuomo RE, French CB, Garland CF, Gorham ED, Grant WB, et al. Sunlight and Vitamin D: necessary for public health. J Am Coll Nutr. 2015;34(4):359–65. https://doi.org/10.1080/07315724.2015.1039866.
• Dominguez LJ, Farruggia M, Veronese N, Barbagallo M. Vitamin D sources, metabolism, and deficiency: available compounds and guidelines for its treatment. Metabolites. 2021;11(4). https://doi.org/10.3390/metabo11040255. This represents a current critical review of vitamin D3 sources, metabolism and deficiency.
• Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res Off J Am Soc Bone Miner Res. 2011;26(3):455–7. https://doi.org/10.1002/jbmr.328. While not recent, this is one of the key papers which initiated the debate of why vitamin D3 IOM levels are majorly underestimated and need revision.
Papadimitriou DT. The big Vitamin D mistake. J Prev Med Public Health. 2017;50(4):278–81. https://doi.org/10.3961/jpmph.16.111.
LeBlanc ES, Zakher B, Daeges M, Pappas M, Chou R. Screening for vitamin D deficiency: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162(2):109–22. https://doi.org/10.7326/M14-1659.
• Moukayed M, Grant WB. Linking the metabolic syndrome and obesity with vitamin D status: risks and opportunities for improving cardiometabolic health and well-being. Diabetes Metab Syndr Obes Targets Ther. 2019;12:1437–47. https://doi.org/10.2147/DMSO.S176933. This paper reviews the role of vitamin D3 in mitigating cardiometabolic risk factors.
L Bishop E, Ismailova A, Dimeloe S, Hewison M, White JH. Vitamin D and immune regulation: antibacterial, antiviral, anti-inflammatory. JBMR Plus. 2021;5(1):e10405. https://doi.org/10.1002/jbm4.10405.
Gilani SJ, Bin-Jumah MN, Nadeem MS, Kazmi I. Vitamin D attenuates COVID-19 complications via modulation of proinflammatory cytokines, antiviral proteins, and autophagy. Expert Rev Anti Infect Ther. 2022;20(2):231–41. https://doi.org/10.1080/14787210.2021.1941871.
Ahmed F. A network-based analysis reveals the mechanism underlying vitamin D in suppressing cytokine storm and virus in SARS-CoV-2 infection. Front Immunol. 2020;11:590459. https://doi.org/10.3389/fimmu.2020.590459.
• Peng MY, Liu WC, Zheng JQ, Lu CL, Hou YC, Zheng CM, et al. Immunological aspects of SARS-CoV-2 infection and the putative beneficial role of Vitamin-D. Int J Mol Sci. 2021;22(10). https://doi.org/10.3390/ijms22105251. This review highlights the important immunomodulatory role of vitamin D3 in SARS-CoV-2 affected individuals.
Lee C. Controversial effects of Vitamin D and related genes on viral infections, pathogenesis, and treatment outcomes. Nutrients. 2020;12(4):962. https://doi.org/10.3390/nu12040962.
Crafa A, Cannarella R, Condorelli RA, Mongioi LM, Barbagallo F, Aversa A, et al. Influence of 25-hydroxy-cholecalciferol levels on SARS-CoV-2 infection and COVID-19 severity: a systematic review and meta-analysis. EClinicalMedicine. 2021;37:100967. https://doi.org/10.1016/j.eclinm.2021.100967.
Israel A, Cicurel A, Feldhamer I, Stern F, Dror Y, Giveon SM, et al. Vitamin D deficiency is associated with higher risks for SARS-CoV-2 infection and COVID-19 severity: a retrospective case-control study. Intern Emerg Med. 2022;17(4):1053–63. https://doi.org/10.1007/s11739-021-02902-w.
Kaufman HW, Niles JK, Kroll MH, Bi C, Holick MF. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PloS one. 2020;15(9):e0239252. https://doi.org/10.1371/journal.pone.0239252.
Sulli A, Gotelli E, Casabella A, Paolino S, Pizzorni C, Alessandri E, et al. Vitamin D and lung outcomes in elderly COVID-19 patients. Nutrients. 2021;13(3):717. https://doi.org/10.3390/nu13030717.
Annweiler C, Beaudenon M, Gautier J, Simon R, Dubee V, Gonsard J, et al. Covid-19 and high-dose VITamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): study protocol for a randomized controlled trial. Trials. 2020;21(1):1031. https://doi.org/10.1186/s13063-020-04928-5.
Annweiler G, Corvaisier M, Gautier J, Dubee V, Legrand E, Sacco G, et al. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study. Nutrients. 2020;12(11):3377. https://doi.org/10.3390/nu12113377.
Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcala Diaz JF, Lopez Miranda J, Bouillon R, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. https://doi.org/10.1016/j.jsbmb.2020.105751.
Abroug H, Maatouk A, Bennasrallah C, Dhouib W, Ben Fredj M, Zemni I, et al. Effect of vitamin D supplementation versus placebo on recovery delay among COVID-19 Tunisian patients: a randomized-controlled clinical trial. Trials. 2023;24(1):123. https://doi.org/10.1186/s13063-023-07114-5.
Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CSC, et al. Effect of a single high dose of Vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325(11):1053–60. https://doi.org/10.1001/jama.2020.26848.
Jevalikar G, Mithal A, Singh A, Sharma R, Farooqui KJ, Mahendru S, et al. Lack of association of baseline 25-hydroxyvitamin D levels with disease severity and mortality in Indian patients hospitalized for COVID-19. Sci Rep. 2021;11(1):6258. https://doi.org/10.1038/s41598-021-85809-y.
• Ghelani D, Alesi S, Mousa A. Vitamin D and COVID-19: an overview of recent evidence. Int J Mol Sci. 2021;22(19). https://doi.org/10.3390/ijms221910559. Excellent current review evaluating the epidemiological studies regarding the association between vitamin D3 and COVID-19.
Alcala-Diaz JF, Limia-Perez L, Gomez-Huelgas R, Martin-Escalante MD, Cortes-Rodriguez B, Zambrana-Garcia JL, et al. Calcifediol treatment and hospital mortality due to COVID-19: a cohort study. Nutrients. 2021;13(6):1760. https://doi.org/10.3390/nu13061760.
Annweiler C, Beaudenon M, Gautier J, Gonsard J, Boucher S, Chapelet G, et al. High-dose versus standard-dose vitamin D supplementation in older adults with COVID-19 (COVIT-TRIAL): a multicenter, open-label, randomized controlled superiority trial. PLoS Med. 2022;19(5):e1003999. https://doi.org/10.1371/journal.pmed.1003999.
Karonova TL, Chernikova AT, Golovatyuk KA, Bykova ES, Grant WB, Kalinina OV, et al. Vitamin D intake may reduce SARS-CoV-2 infection morbidity in health care workers. Nutrients. 2022;14(3):505. https://doi.org/10.3390/nu14030505.
• Shah K, Varna VP, Sharma U, Mavalankar D. Does vitamin D supplementation reduce COVID-19 severity?: a systematic review. QJM: monthly journal of the Association of Physicians. 2022;115(10):665–72. https://doi.org/10.1093/qjmed/hcac040. Excellent current review evaluating the epidemiological studies regarding the association between vitamin D3 and COVID-19.
Wenzel J, Schwaninger M. How COVID-19 affects microvessels in the brain. Brain J Neurol. 2022;145(7):2242–4. https://doi.org/10.1093/brain/awac211.
Lee MH, Perl DP, Nair G, Li W, Maric D, Murray H, et al. Microvascular Injury in the Brains of Patients with Covid-19. N Engl J Med. 2021;384(5):481–3. https://doi.org/10.1056/NEJMc2033369.
• Marek K, Cichon N, Saluk-Bijak J, Bijak M, Miller E. The role of vitamin D in stroke prevention and the effects of its supplementation for post-stroke rehabilitation: a narrative review. Nutrients. 2022;14(13). https://doi.org/10.3390/nu14132761. This review beautifully presents current findings into the capability of vitamin D3 in in brain repair and function even after injury. Vitamin D3 therefore can have a long term therapeutic potential in long COVID-19 brain related dysfunctions.
Aspell N, Lawlor B, O’Sullivan M. Is there a role for vitamin D in supporting cognitive function as we age? Proc Nutr Soc. 2018;77(2):124–34. https://doi.org/10.1017/S0029665117004153.
Zhao W, Zhu DM, Li S, Cui S, Jiang P, Wang R, et al. The reduction of vitamin D in females with major depressive disorder is associated with worse cognition mediated by abnormal brain functional connectivity. Prog Neuropsychopharmacol Biol Psychiatry. 2022;118:110577. https://doi.org/10.1016/j.pnpbp.2022.110577.
DeLuca GC, Kimball SM, Kolasinski J, Ramagopalan SV, Ebers GC. Review: the role of vitamin D in nervous system health and disease. Neuropathol Appl Neurobiol. 2013;39(5):458–84. https://doi.org/10.1111/nan.12020.
Russo C, Valle MS, Casabona A, Spicuzza L, Sambataro G, Malaguarnera L. Vitamin D impacts on skeletal muscle dysfunction in patients with COPD promoting mitochondrial health. Biomedicines. 2022;10(4):898. https://doi.org/10.3390/biomedicines10040898.
• Tomlinson PB, Joseph C, Angioi M. Effects of vitamin D supplementation on upper and lower body muscle strength levels in healthy individuals. A systematic review with meta-analysis. J Sci Med Sport. 2015;18(5):575–80. https://doi.org/10.1016/j.jsams.2014.07.022. A systematic review demonstrating the important role of vitamin D3 in muscle regeneration. Muscle repair and regeneration is important in tackling myalgia, fatigue and other related complications of long COVID-19.
Latham CM, Brightwell CR, Keeble AR, Munson BD, Thomas NT, Zagzoog AM, et al. Vitamin D promotes skeletal muscle regeneration and mitochondrial health. Front Physiol. 2021;12:660498. https://doi.org/10.3389/fphys.2021.660498.
Tan CW, Ho LP, Kalimuddin S, Cherng BPZ, Teh YE, Thien SY, et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrition. 2020;79:111017. https://doi.org/10.1016/j.nut.2020.111017.
• McCullough PJ, Lehrer DS, Amend J. Daily oral dosing of vitamin D3 using 5000 TO 50,000 international units a day in long-term hospitalized patients: insights from a seven year experience. J Steroid Biochem Mol Biol. 2019;189:228–39. https://doi.org/10.1016/j.jsbmb.2018.12.010. This study clearly emphasizes that higher vitamin D3 doses can be administered to improve health outcomes and reduce hospitalization in patients without concerns of putative vitamin D side-effects.
Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–3. https://doi.org/10.1093/ajcn/72.3.690.
Grant WB, Boucher BJ, Al Anouti F, Pilz S. Comparing the evidence from observational studies and randomized controlled trials for nonskeletal health effects of vitamin D. Nutrients. 2022;14(18):3811.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interests or Disclosures
None to declare.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moukayed, M. A Narrative Review on the Potential Role of Vitamin D3 in the Prevention, Protection, and Disease Mitigation of Acute and Long COVID-19. Curr Nutr Rep 12, 215–223 (2023). https://doi.org/10.1007/s13668-023-00471-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-023-00471-2