Abstract
Purpose of the Review
The endocannabinoid system (ENS) has emerged as an important factor in food intake and may have implications for nutrition research. The objective of the current report is to summarise the available evidence on the ENS and eating behaviour from both animal and human studies.
Recent Findings
The literature reviewed demonstrates a clear link between the ENS and eating behaviours. Overall, studies indicate that 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamine (AEA) via cannabinoid receptor-1 (CNR1) binding may stimulate hunger and food intake while oleylethanolamide (OEA) may inhibit hunger. Mechanisms of these associations are not yet well understood, although the evidence suggests that there may be interactions with other physiological systems to consider. Most studies have been conducted in animal models, with few human studies available.
Summary
Additional research is warranted among human populations into the ENS and eating behaviour. Evaluation of relationships between variation in ENS genes and dietary outcomes is an important area for investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
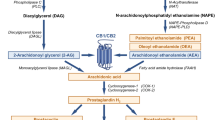
The endocannabinoid system (ENS) is a signalling system consisting of endocannabinoids (ECs), endogenous compounds derived from long-chain polyunsaturated fatty acids, and cannabinoid receptors which serve as binding sites for ECs to modulate various regulatory reactions in the body [1,2,3]. In 1990, in searching for a mechanism explaining the therapeutic effects of cannabis, Matsuda et al. isolated a first G-protein couple receptor that reacted with active compounds in cannabis in rat brain, cannabinoid receptor-1 (CNR1) [4]. This was quickly followed by the discovery of cannabinoid receptor-2 (CNR2) in 1993, not in rat brain as seen for CNR1, but in rat spleen [5]. This not only helped further the understanding of the binding of active cannabis compounds, but also paved the way to the discovery of ECs that bind these receptors, of which the first to be discovered were N-arachidonoylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG), both derived from arachidonic acid [2]. Aside from the latter, which are the most common, there exist many other ECs including N-arachidonoyldopamine (NADA), 2-arichidonoylglycerylether (noladin ether) and O-arachidonoylethanolamine [6]. Evidence shows that AEA and 2-AG function through retrograde signalling where they are synthesised de novo based on intracellular calcium concentrations and activate CNR1 to inhibit neurotransmitter release [2, 7].
We now know that CNR1 is nearly ubiquitous in the human body, with expression in the central nervous system and many other tissues and systems (i.e. liver, reproductive system, gastrointestinal tract, skeletal muscles, the cardiovascular system) [7]. CNR2, on the other hand, is mainly expressed in immune cells and other peripheral tissues, but not in the central nervous system [7]. The ENS also contains endocannabinoid-like compounds—N-acylethanolamines (NEAs), e.g. palmitoylethanolamide (PEA), oleylethanolamide (OEA) and 2-monoacylglycerols (2-MAGs)—which are structurally similar to ECs but they do not bind to cannabinoid receptors, although they are metabolised through pathways involving the same enzymes [8, 9]. Nonetheless they play an important role in the ENS and have been linked to appetite and food intake [8]. Although the exact mechanisms of action of ECs and EC-like compounds are not yet fully understood, certain key enzymes have been highlighted such as N-acyl-phosphatidylethanolamines (NAPE)-hydrolysing phospholipase D involved in the synthesis of these compounds and fatty acid amide hydrolase (FAAH) involved in their degradation [9]. It is also recognised that ECs and EC-like compounds interact with receptors other than CNR1 and CNR2 which further contributes to the versatility of their biological functions [9].
Given the global rise in obesity and metabolic disorders, it is important to explore the various factors that may contribute to energy balance in humans. The ENS has emerged as an important actor in food intake and hedonic eating [10, 11]. Equally as important is understanding the role of genetic variation in the ENS and its impact on food behaviour and preference to identify vulnerable groups who may be most susceptible to hedonic eating and thus may need different diet therapy approaches for regulating eating behaviour. The objective of the current report is to summarise the available evidence on the ENS and eating behaviour from multiple modes of inquiry, including both animal and human studies.
Methods
PubMed was searched for studies using keywords relating to the “endocannabinoid system” and “eating behaviour”. The search strategy focused on using “Medical subject headings” (MeSH terms) without language restriction as follows: (Endocannabinoid* [tiab] OR “endocannabinoid system” [tiab] OR cannabinoid* [tiab] OR “endocannabinoids” [MeSH Terms] OR “Cannabinoids” [MESH] OR “Cannabinoid Receptor Modulators” [MESH] OR “cannabinoid receptor agonists” [MeSH Terms]) AND (“eating behavior*” [tiab] OR “eating behaviour*” [tiab] OR appetite [tiab] OR “food intake” [tiab] OR “food consumption” [tiab] OR craving [tiab] OR “eating habit*” [tiab] OR “feeding behavior” [MeSH Terms] OR “feeding behavior” [tiab]). The search was run in January 2022 and was not restricted to any time period. Title and abstract screening was carried out in the first phase of inclusion, and this was followed by full-text screening. Additional articles were included through a search of reference lists of included studies. Screening was performed by NAV and DEN provided consultation to resolve uncertainties. Exclusion criteria included retrievals that were not primary research articles (e.g. review articles, perspectives, editorials), articles that did not consider eating behaviour, and articles that did not consider the ENS.
Results
The search strategy retrieved a total of 294 articles and 3 articles were added from manual reference lists (Fig. 1). Upon completion of article screening, 38 articles were eligible to be included in the present review. Thirty studies were conducted in animal models (Table 1) and eight studies were conducted in humans (Table 2).
Endocannabinoid Compounds and Eating Behaviour
Various studies have previously investigated the link between ECs and food intake and preferences in both animal models and humans. In a recent study, researchers presented Drosophila melanogaster with foods containing ECs, including AEA, 2-AG, 2-linoleoyl glycerol (2-LG) and arachidonic acid (AA), as well as phytocannabinoids (from Cannabis sativa) and synthetic cannabinoids (mainly formulated for pharmaceutical purposes) [12]. Liquid food consumption containing sucrose and yeast with cannabinoids was compared to control liquid feed. Authors found that the flies had a significantly stronger preference for food containing higher concentrations of 2-AG (for up to 4 days) and AEA (sustained throughout experiment) [12]. Foods containing phytocannabinoids and certain synthetic cannabinoids were also preferred. Interestingly, the authors also found that AEA and 2-AG suppressed food intake, and that AEA significantly increases survival rate in starving fruit flies, potentially by reducing lipid metabolism as demonstrated by higher levels of triglycerides in flies that previously consumed EC-containing foods [12]. The main caveat to consider with this study, as explained by the authors, is that CNR1 and CNR2 are not expressed in Drosophila; thus, the effects of ECs observed may not be applicable in humans or other animal models that express these receptors. However, this may provide some clues into reactions to ECs in reduced expression of CNR1 and CNR2.
A previous study found an inverse association between ECs and hedonic eating in humans. Normal weight, healthy subjects that had reached satiation were given palatable foods of their preference (i.e. traditional cakes) that they could consume ad libitum in an initial session which was followed by a second session where they were provided the same amount of non-preferred isocaloric foods similar in nutritional value (i.e. bread, milk, butter) that could also be consumed ad libitum and combined as desired (i.e. bread and butter, bread and milk) [13]. It was found that 2-AG levels were significantly higher before and during hedonic eating of preferred foods when compared to eating non-preferred foods and that 2-AG levels were significantly positively correlated with plasma ghrelin levels [13]. This has also been tested in subjects with binge-eating disorder (BED) and obesity. In subjects with BED, 2-AG levels were not significantly different between preferred and non-preferred foods, but rather AEA levels significantly increased after eating preferred foods, while in obese individuals 2-AG significantly increased before eating preferred foods and AEA levels decreased [14, 15].
There is also evidence of and interaction between the ENS and opioid system in rats. In a 2015 study, Parker et al. administered varying doses of u-opioid agonist D-Ala2, NMe-Phe4, Glyol5-enkephalin (DAMGO) and 2-AG in nucleus accumbens, involved in the hedonic food response, of Sprague–Dawley rats fed a high-fat diet [16]. The authors found that the combined effect of high-dose DAMGO and subthreshold dose of 2-AG resulted in significantly higher observations of food-seeking behaviour and food-related locomotor activity when compared to high DAMGO administration alone [16]. However, the effect on overall consumption (grams consumed) was nonsignificant, although there was a trend towards increased consumption [16]. Nevertheless, the authors conclude a potential significant interaction between the opioid and endocannabinoid system on high-fat feeding [16].
The hyperphagic effects of 2-AG have also been detected in layer-type chicken where administration of 2-AG resulted in a significantly higher food intake and SR141716A, a CNR1 antagonist, resulted in inhibited appetite [17, 18]. Additionally, another study also found that administration of AEA in Wistar rats provided with sucralose solutions resulted in a significantly higher expression of ΔFosB in the nucleus accumbens (compared to rats that were not administered AEA), which would also indicate a potential change in the food reward system due to the administration of AEA [19]. However, similarly to the latter study, the authors did not show any impact on the overall intake of sucralose in rats given AEA [19]. The importance of AEA in eating is supported by a study led in Wistar rats that were administered AM404, GW6471 and SR141716A, drugs known to interfere with metabolism of ECs [20]. In this study, administration of AM404 and PPAR-α antagonist, GW6471, led to increased food intake while SR141716A resulted in suppressed appetite and counteracted the appetite stimulating effects of AM404 [20]. This may suggest that AEA stimulates appetite by binding to CNR1 but when the activity of this receptor is inhibited, hunger is suppressed. This had also previously been demonstrated in a study wherein male Wistar rats were injected with AEA which stimulated food intake, while SR141716A inhibited food intake [21]. Additionally, OEA administration also increased feeding, with a possible synergistic effect with SR141716A [21]. The 2-AG compound may also be related to food preference via its interaction with CNR1 as shown in male mice fed a high-fat diet with resulted in increased 2-AG plasma levels and a subsequent increased preference for high-fat diet [22, 23].
There may also be a link between ECs and preference for sweet taste. In a study, Sprague–Dawley rats were injected with doses of AEA and a control vehicle in their nucleus accumbens 48 h apart to identify whether AEA had an impact on preference for sucrose, dislike of quinine (bitter taste) and overall food intake [24]. It was found that upon AEA administration sucrose preferences were up to two times higher than when control vehicle was administered, and that time spent eating and eating bouts were more than doubled, resulting in an overall sixfold increase in intake [24]. AEA had no effect on reactions to bitter taste [24]. There is also evidence suggesting that EC levels may vary by fed state. In one of the first studies to investigate EC variations in the brain, Kirkham et al. found that fasting led to higher anandamide and 2-AG levels in the brain of Lister hooded rats compared to rats fed ad libitum, while eating resulted in declining 2-AG levels [25•]. This study also further validates the findings of previous studies that 2-AG administration increases food intake while SR141716A has an anorectic effect.
Differential effects of ECs may occur depending on variation in individual taste sensitivity to 6-n-propylthiouracil (PROP). PROP taste sensitivity is associated with a detection of bitter taste in foods (or super tasters), while those who are not sensitive to PROP (or non-tasters) have been found to have a preference for high-fat, energy-dense foods [26]. In a study, individuals were groups by PROP sensitivity (super tasters or non-tasters) and were asked to complete a Three-Factor Eating Questionnaire as well as to provide a blood sample for a plasma EC profile [26]. Significant associations were found in non-tasters only where higher OEA plasma levels were significantly correlated with lower perceived hunger, while higher plasma AEA levels were correlated with higher restraint and lower perceived hunger [26]. Surprisingly, it was found that plasma AEA and 2-AG levels were significantly lower in non-tasters, although the inverse may have been expected due to the association with higher energy diets and previous studies presented here that have found higher AEA and 2-AG associated with hyperphagic effects [26]. In fact, a previous study has found that 2-AG, along with another endocannabinoid, noladin ether, is significantly associated with hyperphagia and increased high-fat feed consumption in rats as compared to high-carbohydrate feed [27]. Additionally, this study highlights that the effects of 2-AG and noladin ether on intake and diet preference can be antagonised by pre-treatment with AM 251 and that noladin ether could have a more potent effect on overall intake than 2-AG [27].
Oleoylethanolamide and Eating Behaviour
Although EC-like compounds resemble ECs, they may have distinct effects on food intake. In particular, research has mainly focused on the effects of OEA. In fact, in free-fed male Wistar rats, OEA capsule administration resulted in appetite inhibition which persisted for over 24 h [28•]. Additionally, OEA may not only have an anorectic effect but it may also result in increased satiety as shown by increased time between meals in rats injected with an adenoviral vector causing them to produce higher amounts of NAPE-phospholipase D which catalyses the synthesis of OEA from NAPE [29]. This resulted in higher intestinal OEA production and increased satiety in rats, which was paired with higher expression of proliferator-activated receptors (PPAR-α) and CD36, both known to be involved in energy balance [29]. In a different rat model, OEA administration resulted in anorectic effects which were not inhibited by CNR1 and CNR2 antagonists SR141716A and SR144528 further reinforcing the notion that OEA does not interact with cannabinoid receptors to modulate eating behaviours [30], thus highlighting the need to look to interaction with other receptors and systems to understand how OEA relates to eating behaviour.
In fact, studies have put forward various potential mechanisms for this relationship. One such study was carried out in mice in which histidine decarboxylase gene (HDC), which codes for an enzyme responsible for the synthesis of histamine, was knocked out. Authors found that lack of histamine release, which was found to increase with OEA levels, resulted in attenuated anorectic effects of OEA [31], thus highlighting a close relationship. Additionally, there is evidence that OEA may enhance glucagon-like peptide 1 (GLP-1) signalling, involved in glucose homeostasis [32]. OEA may also interact with extendin-4 (Ex4), a GLP-1 receptor agonist, to enhance weight loss in obese mice in a synergistic manner, despite having no impact on the hypophagic effects of GLP-1 and Ex4 [32]. However, no effect of OEA on hypophagic it has also been elucidated that OEA may act by upregulating the OEA-specific G protein coupled receptor 119 (GPR119), which has been previously identified as a mediator in the anorectic effects of OEA, and FAAH, which is responsible for the degradation and cell uptake of ECs such as 2-AG which may increase food intake as previously discussed [33, 34].
Cannabinoid Receptors, Fatty Acid Amide Hydrolase and Eating Behaviours
In both animal and human studies, cannabinoid receptors, particularly CNR1, and FAAH have been found to modulate food intake. In goldfish, it has been found that food deprivation leads to increased CNR1 expression in the brain, which is lowered upon refeeding or upon administration of AEA [35]. In rats, it has also been shown that CNR1 receptor antagonists, AM251, AM404, O-2050 and SR141716A, significantly suppress food intake behaviour regardless of macronutrient content of feed [36,37,38]. It has been posited that CNR1 antagonists may act through both inducing satiation and reducing reward mechanisms associated with food [39]. Conversely, it has also been suggested that AM251 may act to reduce response to palatable foods such as chocolate-flavoured feed in rats, rather than decrease overall intake [40]. On the other hand, CNR1 stimulation enhances food intake [38].
CNR1 may also be involved in fat taste perception as a previous study demonstrated a reduced preference for fat among CNR1 knockout mice [41]. This is also supported in a different study where administration of O-2050, a CNR1 antagonist, also resulted in reduced dietary fat preference in mice which had previously been fed a high-fat diet for 2 weeks and had developed a preference for this diet type [42]. However, a study in Wistar rats has also shown that activation of CNR1 via administration of CNR1 agonist N-[2]-5Z,8Z,11Z,14Z-eicosatetraenamide (ACEA) resulted in increased preference for carbohydrates when rats were also offered protein and fat feed [43]. This effect was counteracted by the administration of the CNR1 antagonist AM251, similarly to what was observed in Deshmukh and Sharma [27]. ACEA also reversed satiation in rats and led to increased feed time [43]. One study also alluded to a potential role of CNR1 in increasing odour detection which may be linked to increased food intake [44].
Furthermore, FAAH has been associated with certain eating patterns. For example, it has been previously shown that FAAH levels decrease in the brains of rats with binge-eating behaviours [45]. This has also been elucidated to in humans wherein higher frequencies of the FAAH 385 C to A single nucleotide polymorphism (SNP) (hereafter rs324420) have been associated with anorexia and bulimia nervosa [46]. Similar associations have also been seen for the CNR1 1359 G to A SNP (hereafter rs1049353) [46]. Additionally, rs324420 has also been associated with increase rates of overweight and obesity in a white and black populations, but not in an Asian population [47]. The rs1049353 SNP has also been associated with increased consumptions of complex carbohydrates and decreased dietary cholesterol and saturated fat [48•]. Despite this, rs1049353 was not found to be associated with weight loss [49].
Conclusion
The literature reviewed in this report demonstrates that there is a clear link between ENS and eating behaviours which has been largely investigated in animal models. Overall, studies seem to indicate that 2-AG and AEA via CNR1 binding may stimulate hunger and food intake while OEA may inhibit hunger. Mechanisms of these associations are not yet well understood, although literature indicates that there may be interactions with other physiological systems to consider. Caruso et al. reported an association between rs1049353 and food group consumption [48•]. The association between rs1049353 and alcohol dependence has previously been studied in a meta-analysis, with no significant associations either [50]. Thus, it is important to continue to investigate this association in different populations given the known importance of FAAH in regulating food intake.
Given the paucity in human studies in this research area, future studies should focus on observing these associations in humans, specifically on whether variation in ENS genes can help explain differences in diet quality, hedonic eating and food intake. Although there appears to be a large pharmaceutical interest in this area to identify substances that target the ENS, such as the CNR1 antagonist rimonabant, and can result in suppressed appetite and weight loss, it is important to also explore the most appropriate diet therapies that consider the role of the ENS in eating behaviour. An additional aspect that this report did not consider is the role of exposures in the food environment, which has become increasingly obesogenic. Ubiquitous cues towards highly palatable foods may impact processes in the ENS and is an area for future research.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Marzo VD. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol. 2006;1–24.
Matias I, Di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol Metab. 2007;18(1):27–37.
Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998;359(1):1–18.
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–4.
Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–5.
Battista N, Di Tommaso M, Bari M, Maccarrone M. The endocannabinoid system: an overview. Front Behav Neurosci. 2012;6:9.
Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3):833.
Kleberg K, Hassing HA, Hansen HS. Classical endocannabinoid-like compounds and their regulation by nutrients. BioFactors. 2014;40(4):363–72.
Fezza F, Bari M, Florio R, Talamonti E, Feole M, Maccarrone M. Endocannabinoids, related compounds and their metabolic routes. Molecules. 2014;19(11):17078–106.
Tarragon E, Moreno JJ. Role of endocannabinoids on sweet taste perception, food preference, and obesity-related disorders. Chem Senses. 2018;43(1):3–16.
Watkins BA, Kim J. The endocannabinoid system: directing eating behavior and macronutrient metabolism. Front Psychol. 2014;5:1506.
He J, Tan AMX, Ng SY, Rui M, Yu F. Cannabinoids modulate food preference and consumption in Drosophila melanogaster. Sci Rep. 2021;11(1):4709.
Monteleone P, Piscitelli F, Scognamiglio P, Monteleone AM, Canestrelli B, Di Marzo V, et al. Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-arachidonoyl-glycerol in healthy humans: a pilot study. J Clin Endocrinol Metab. 2012;97(6):E917–24.
Monteleone AM, Di Marzo V, Monteleone P, Dalle Grave R, Aveta T, Ghoch ME, et al. Responses of peripheral endocannabinoids and endocannabinoid-related compounds to hedonic eating in obesity. Eur J Nutr. 2016;55(4):1799–805.
Monteleone AM, Piscitelli F, Dalle Grave R, El Ghoch M, Di Marzo V, Maj M, et al. Peripheral endocannabinoid responses to hedonic eating in binge-eating disorder. Nutrients. 2017;9(12).
Parker KE, McCall JG, McGuirk SR, Trivedi S, Miller DK, Will MJ. Effects of co-administration of 2-arachidonylglycerol (2-AG) and a selective µ-opioid receptor agonist into the nucleus accumbens on high-fat feeding behaviors in the rat. Brain Res. 2015;1618:309–15.
Keyshams N, Zendehdel M, Babapour V, Baghbanzadeh A. Cannabinoid-glutamate interactions in the regulation of food intake in neonatal layer-type chicks: role of glutamate NMDA and AMPA receptors. Vet Res Commun. 2016;40(2):63–71.
Alizadeh A, Zendehdel M, Babapour V, Charkhkar S, Hassanpour S. Role of cannabinoidergic system on food intake in neonatal layer-type chicken. Vet Res Commun. 2015;39(2):151–7.
Salaya-Velazquez NF, López-Muciño LA, Mejía-Chávez S, Sánchez-Aparicio P, Domínguez-Guadarrama AA, Venebra-Muñoz A. Anandamide and sucralose change ΔFosB expression in the reward system. NeuroReport. 2020;31(3):240–4.
Reyes-Cabello C, Alen F, Gómez R, Serrano A, Rivera P, Orio L, et al. Effects of the anandamide uptake blocker AM404 on food intake depend on feeding status and route of administration. Pharmacol Biochem Behav. 2012;101(1):1–7.
Gómez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, et al. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22(21):9612–7.
Higuchi S, Ohji M, Araki M, Furuta R, Katsuki M, Yamaguchi R, et al. Increment of hypothalamic 2-arachidonoylglycerol induces the preference for a high-fat diet via activation of cannabinoid 1 receptors. Behav Brain Res. 2011;216(1):477–80.
Higuchi S, Irie K, Yamaguchi R, Katsuki M, Araki M, Ohji M, et al. Hypothalamic 2-arachidonoylglycerol regulates multistage process of high-fat diet preferences. PLoS One. 2012;7(6).
Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32(11):2267–78.
• Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136(4):550–7. (In this study, the effect of feeding and fasting on brain endocannabinoid levels (N-arachidonoylethanolamine and 2-arachidonoylglycerol in particular) was studied in rats. This is one of the first studies to provide evidence supporting the role of the endocannabinoid system in eating behaviour.)
Tomassini Barbarossa I, Carta G, Murru E, Melis M, Zonza A, Vacca C, et al. Taste sensitivity to 6-n-propylthiouracil is associated with endocannabinoid plasma levels in normal-weight individuals. Nutrition. 2013;29(3):531–6.
Deshmukh RR, Sharma PL. Stimulation of accumbens shell cannabinoid CB(1) receptors by noladin ether, a putative endocannabinoid, modulates food intake and dietary selection in rats. Pharmacol Res. 2012;66(3):276–82.
• Oveisi F, Gaetani S, Eng KT, Piomelli D. Oleoylethanolamide inhibits food intake in free-feeding rats after oral administration. Pharmacol Res. 2004;49(5):461–6. (In this study, oral administration of oleoylethanolamide resulted in reduced food intake in rats. This study suggests that oral oleoylethanolamide can induce a state of satiety and increase time between meals.)
Fu J, Kim J, Oveisi F, Astarita G, Piomelli D. Targeted enhancement of oleoylethanolamide production in proximal small intestine induces across-meal satiety in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R45-50.
Rodríguez de Fonseca F, Navarro M, Gómez R, Escuredo L, Nava F, Fu J, et al. An anorexic lipid mediator regulated by feeding. Nature. 2001;414(6860):209–12.
Provensi G, Coccurello R, Umehara H, Munari L, Giacovazzo G, Galeotti N, et al. Satiety factor oleoylethanolamide recruits the brain histaminergic system to inhibit food intake. Proc Natl Acad Sci USA. 2014;111(31):11527–32.
Brown JD, McAnally D, Ayala JE, Burmeister MA, Morfa C, Smith L, et al. Oleoylethanolamide modulates glucagon-like peptide-1 receptor agonist signaling and enhances exendin-4-mediated weight loss in obese mice. Am J Physiol Regul Integr Comp Physiol. 2018;315(4):R595-r608.
Thabuis C, Destaillats F, Landrier JF, Tissot-Favre D, Martin JC. Analysis of gene expression pattern reveals potential targets of dietary oleoylethanolamide in reducing body fat gain in C3H mice. J Nutr Biochem. 2010;21(10):922–8.
Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3(3):167–75.
Cottone E, Guastalla A, Pomatto V, Campantico E, Di Marzo V, Franzoni M. Goldfish CB1 mRNA expression is affected by fasting and anandamide administration. NeuroReport. 2009;20(6):595–9.
McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, et al. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14(8):583–8.
Gardner A, Mallet PE. Suppression of feeding, drinking, and locomotion by a putative cannabinoid receptor ‘silent antagonist.’ Eur J Pharmacol. 2006;530(1–2):103–6.
Gianessi CA, Groman SM, Taylor JR. Bi-directional modulation of food habit expression by the endocannabinoid system. Eur J Neurosci. 2019;49(12):1610–22.
Thompson EE, Jagielo-Miller JE, Vemuri VK, Makriyannis A, McLaughlin PJ. CB1 antagonism produces behaviors more consistent with satiety than reduced reward value in food-maintained responding in rats. J Psychopharmacol. 2016;30(5):482–91.
Droste SM, Saland SK, Schlitter EK, Rodefer JS. AM 251 differentially effects food-maintained responding depending on food palatability. Pharmacol Biochem Behav. 2010;95(4):443–8.
Brissard L, Leemput J, Hichami A, Passilly-Degrace P, Maquart G, Demizieux L, et al. Orosensory detection of dietary fatty acids is altered in CB1R(-/-) mice. Nutrients. 2018;10(10).
Higuchi S, Irie K, Mishima S, Araki M, Ohji M, Shirakawa A, et al. The cannabinoid 1-receptor silent antagonist O-2050 attenuates preference for high-fat diet and activated astrocytes in mice. J Pharmacol Sci. 2010;112(3):369–72.
Escartín-Pérez RE, Cendejas-Trejo NM, Cruz-Martínez AM, González-Hernández B, Mancilla-Díaz JM, Florán-Garduño B. Role of cannabinoid CB1 receptors on macronutrient selection and satiety in rats. Physiol Behav. 2009;96(4–5):646–50.
Soria-Gómez E, Bellocchio L, Reguero L, Lepousez G, Martin C, Bendahmane M, et al. The endocannabinoid system controls food intake via olfactory processes. Nat Neurosci. 2014;17(3):407–15.
Pucci M, Micioni Di Bonaventura MV, Zaplatic E, Bellia F, Maccarrone M, Cifani C, et al. Transcriptional regulation of the endocannabinoid system in a rat model of binge-eating behavior reveals a selective modulation of the hypothalamic fatty acid amide hydrolase gene. Int J Eat Disord. 2019;52(1):51–60.
Monteleone P, Bifulco M, Di Filippo C, Gazzerro P, Canestrelli B, Monteleone F, et al. Association of CNR1 and FAAH endocannabinoid gene polymorphisms with anorexia nervosa and bulimia nervosa: evidence for synergistic effects. Genes Brain Behav. 2009;8(7):728–32.
Sipe JC, Waalen J, Gerber A, Beutler E. Overweight and obesity associated with a missense polymorphism in fatty acid amide hydrolase (FAAH). Int J Obes. 2005;29(7):755–9.
• Caruso MG, Gazzerro P, Notarnicola M, Cisternino AM, Guerra V, Misciagna G, et al. Cannabinoid type 1 receptor gene polymorphism and macronutrient intake. J Nutrigenet Nutrigenomics. 2012;5(6):305–13. (In this study, a Southern Italian cohort older than 65 years with known rs1049353 (or CNR1 1359 G to A) genotype completed a food frequency questionnaire to assess eating habits. The presence of the rs1049353 single nucleotide polymorphism was linked to micronutrient intake.)
de Luis DA, Aller R, Izaola O, Díaz Soto G, López Gómez JJ, Gómez Hoyos E, et al. Effects of a high-protein/low-carbohydrate versus a standard hypocaloric diet on weight and cardiovascular risk factors during 9 months: role of a genetic variation in the cannabinoid receptor gene (CNR1) (G1359A polymorphism). Ann Nutr Metab. 2015;66(2–3):125–31.
Pabalan N, Chaweeborisuit P, Tharabenjasin P, Tasanarong A, Jarjanazi H, Eiamsitrakoon T, et al. Associations of CB1 cannabinoid receptor (CNR1) gene polymorphisms with risk for alcohol dependence: evidence from meta-analyses of genetic and genome-wide association studies. Medicine. 2021;100(43): e27343.
Acknowledgements
We acknowledge the support of the Government of Canada’s New Frontiers in Research Fund (NFRF), [NFRFE-2018-00459].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
The authors declare they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nutrition and the Brain
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aguilera Vasquez, N., Nielsen, D.E. The Endocannabinoid System and Eating Behaviours: a Review of the Current State of the Evidence. Curr Nutr Rep 11, 665–674 (2022). https://doi.org/10.1007/s13668-022-00436-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-022-00436-x