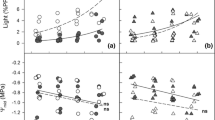
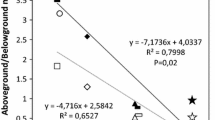
Abstract
•Context
Tree species differ largely in their capability to produce characteristic shade leaves with effective morphological and physiological acclimation to low light.
•Aims
By examining the sun/shade leaf differentiation in leaf morphology, foliar nitrogen and photosynthetic capacity in five temperate tree species of different successional status, we aimed at identifying those leaf traits that determine the development of a typical shade crown with low light-acclimated leaves.
•Methods
Leaf morphology, foliar N content, photosynthetic capacity (V cmax, J max and A max) and leaf dark respiration (R d) were measured in the canopies of 26 adult trees of Fraxinus, Acer, Carpinus, Tilia and Fagus species.
•Results
Six traits (the sun/shade leaf differentiation in specific leaf area, leaf size, A max per leaf area or per mass, photosynthetic N use efficiency and R d) were found to characterise best the degree of low light acclimation in shade leaves. All five species exhibited certain modifications in leaf morphology and/or physiology in response to low light; Fagus sylvatica showed the highest and Fraxinus excelsior the lowest shade leaf acclimation.
•Conclusions
Our results indicate that the five early/mid- to late-successional species have developed species-specific low light acclimation strategies in their shade crowns which differ in terms of the relative importance of leaf morphological and physiological acclimation.





Similar content being viewed by others
References
Ball JT, Woodrow IE, Berry JA (1987) A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. In: Biggins J (ed) Progress in photosynthesis research IV. Martinus Nijhof, Dordrecht, pp 221–234
Bassow SL, Bazzaz FA (1997) Intra- and inter-specific variation in canopy photosynthesis in a mixed deciduous forest. Oecologia 109:507–515
Bazzaz FA (1979) Physiological ecology of plant succession. Annu Rev Ecol Syst 10:351–371
Brooks A, Farquhar GD (1985) Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase oxygenase and the rate of respiration in the light—estimates from gas-exchange measurements on spinach. Planta 165:397–406
Coste S, Roggy J-C, Sonnier G, Dreyer E (2010) Similar irradiance-elicited plasticity of leaf traits in saplings of 12 tropical rainforest tree species with highly different leaf mass-to-area ratio. Funct Plant Biol 37:1–14
dePury DGG, Farquhar GD (1997) Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant Cell Environ 20:537–557
Ellenberg H, Leuschner C (2010) Vegetation Mitteleuropas mit den Alpen. UTB, Stuttgart
Ellsworth DS, Reich PB (1993) Canopy structure and vertical patterns of photosynthesis and related leaf traits in a deciduous forest. Oecologia 96:169–178
Falster DS, Westoby M (2003) Leaf size and angle vary widely across species: what consequences for light interception? New Phytol 158:509–525
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Fay MP, Shaw PA (2010) Exact and asymptotic weighted logrank tests for interval censored data: the interval R package. J Stat Softw 36:1–34
Fleck S (2002) Integrated analysis of relationships between 3D-structure, leaf photosynthesis, and branch transpiration of mature Fagus sylvatica and Quercus petraea trees in a mixed forest stand. PhD thesis, University of Bayreuth, Bayreuth, Germany
Frazer GW, Trofymow JA, Lertzman KP (1999) Canopy openness and leaf area in chronosequences of coastal temperate rainforests. Can J For Res 30:239–256
Gregor T, Seidling W (1997) 50 years’ succession on a woodland clearing in the mountain area of eastern Hesse. Forstwiss Centrbl 116:218–231
Hallik L, Niinemets U, Wright IJ (2009) Are species shade and drought tolerance reflected in leaf-level structural and functional differentiation in Northern Hemisphere temperate woody flora? New Phytol 184:257–274
Harley PC, Tenhunen JD (1991) Modeling the photosynthetic response of C3 leaves to environmental factors. In: Boote KJ, Loomis RS (eds) Modeling crop photosynthesis—from biochemistry to canopy. American Society of Agronomy and Crop Science Society of America, Madison, pp 17–39
Hölscher D (2004) Leaf traits and photosynthetic parameters of saplings and adult trees of co-existing species in a temperate broad-leaved forest. Basic Appl Ecol 5:163–172
Jones TA, Thomas SC (2007) Leaf-level acclimation to gap creation in mature Acer saccharum trees. Tree Physiol 27:281–290
Köcher P, Gebauer T, Horna V, Leuschner C (2009) Leaf water status and stem xylem flux in relation to soil drought in five temperate broad-leaved tree species with contrasting water use strategies. Ann For Sci 66:101
Kutsch WL, Herbst M, Vanselow R, Hummelshoj P, Jensen NO, Kappen L (2001) Stomatal acclimation influences water and carbon fluxes of a beech forest in northern Germany. Basic Appl Ecol 2:265–281
Larcher W (1994) Ökophysiologie der Pflanzen. UTB, Stuttgart
Leuzinger S, Zotz G, Asshoff R, Körner C (2005) Responses of deciduous forest trees to severe drought in Central Europe. Tree Physiol 25:641–650
Liburnau, H. Ritter Lorenz v. Hrg (1908) Eckert-Lorenz Lehrbuch der Forstwirtschaft für Waldbau und Försterschulen Band 3, Verlag der k.u.k. Hofbuchndlung T.W. Frick, Wien
Lichtenthaler HK, Buschmann C, Döll M, Fietz H-J, Bach T, Kozel U, Meier D, Rahmsdorf U (1981) Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth Res 2:115–141
Masarovicova E, Stefancik L (1990) Some ecophysiological features in sun and shade leaves of tall beech trees. Biol Plant 32:374–387
Montpied P, Granier A, Dreyer E (2009) Seasonal time-course of gradients of photosynthetic capacity and mesophyll conductance to CO2 across a beech (Fagus sylvatica L.) canopy. J Exp Bot 60:2407–2418
Niinemets U (2007) Photosynthesis and resource distribution through plant canopies. Plant Cell Environ 30:1052–1071
Niinemets U (2010) A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol Res 25:693–714
Niinemets U, Kull O, Tenhunen JD (1998) An analysis of light effects on foliar morphology, physiology, and light interception in temperate deciduous woody species of contrasting shade tolerance. Tree Physiol 18:681–696
Ogren E, Evans JR (1993) Photosynthetic light-response curves. 1. The influence of CO2 partial-pressure and leaf inversion. Planta 189:182–190
Popma J, Bongers F, Werger MJA (1992) Gap-dependence and leaf characteristics of trees in a tropical lowland rain forest in Mexico. Oikos 63:207–214
R Development Core Team (2008) R: a language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org. Accessed 15 May 2012
Schulze ED (1970) Der CO2-Gaswechsel der Buche (Fagus sylvatica L.) in Abhängigkeit von den Klimafaktoren im Freiland. Flora 159:177–232
Sims DA, Gebauer RLE, Pearcy RW (1994) Scaling sun and shade photosynthetic acclimation of Alocasia macrorrhiza to whole-plant performance.2. Simulation of carbon balance and growth at different photon flux densities. Plant Cell Environ 17:889–900
Tholen D, Zhu XG (2011) The mechanistic basis of internal conductance: a theoretical analysis of mesophyll cell photosynthesis and CO2 diffusion. Plant Physiol 156:90–105
Thornley JHM (2002) Instantaneous canopy photosynthesis: analytical expressions for sun and shade leaves based on exponential light decay down the canopy and an acclimated non-rectangular hyperbola for leaf photosynthesis. Ann Bot 89:451–458
Urban O, Kosvancova M, Marek MV, Lichtenthaler HK (2007) Induction of photosynthesis and importance of limitations during the induction phase in sun and shade leaves of five ecologically contrasting tree species from the temperate zone. Tree Physiol 27:1207–1215
Valladares F, Niinemets U (2008) Shade tolerance, a key plant feature of complex nature and consequences. Annu Rev Ecol Evol Syst 39:237–257
Valladares F, Chico JM, Aranda I, Balaguer L, Dizengremel P, Manrique E, Dreyer E (2002) The greater seedling high-light tolerance of Quercus robur over Fagus sylvatica is linked to a greater physiological plasticity. Trees 16:395–403
von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387
Wallenda T, Stober C, Hoegbom L, Schinkel H, Georg E, Hoegberg P, Read DJ (2000) Nitrogen uptake processes in roots and mycorrhizas. In: Schulze ED (ed) Carbon and nitrogen cycling in European forest ecosystems. Springer, Berlin, pp 122–143
Walters RG (2005) Towards an understanding of photosynthetic acclimation. J Exp Bot 56:435–447
Acknowledgments
We thank all members of the Göttingen Experimental Botanical Garden (particularly Dirk Deilke and Ulrich Werder) and the student helpers for their support in operating the lifter in the forest. The authors thank the administration of Hainich National Park (especially Jens Wilhelm) for the collaboration and granting access to the forest sites, Paul Köcher for the fruitful cooperation, Professor Zucchini (University of Göttingen) for advice in the statistical analysis and Christina Langenbruch for comments on the manuscript.
Funding
This research was conducted in the framework of ‘Graduiertenkolleg 1086: The Role of Biodiversity for Biogeochemical Cycles and Biotic Interactions in Temperate Deciduous Forests’. The financial support granted by DFG is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Erwin Dreyer
Contribution of the co-authors
SF developed the study design, NL conducted the research, NL with support from SF conducted the analysis, and CL, NL and SF wrote the paper.
Rights and permissions
About this article
Cite this article
Legner, N., Fleck, S. & Leuschner, C. Low light acclimation in five temperate broad-leaved tree species of different successional status: the significance of a shade canopy. Annals of Forest Science 70, 557–570 (2013). https://doi.org/10.1007/s13595-013-0298-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13595-013-0298-4