Abstract
Organisms live within cycles of birth, growth, and reproduction, but life cycles also include decline and death. Here, we focus on the process of colony decline and death in “hopelessly queenless” honey bee colonies (Apis mellifera). In addition, we tracked the parasitic mite, Varroa destructor, to understand how mite populations change during colony decline, and the implications of colony decline on mite transmission. To address these knowledge gaps, we established four hopelessly queenless colonies in observation hives and tracked their bee and mite populations until death. Hopelessly queenless colonies persisted for 2–3 months (86 ± 19 days), with a long-tailed survival distribution (50% of bees dead by day 25; 95% by day 74). In two of the four colonies, the mites outlived the bees by up to 48 h; in one colony the bees outlived the mites by 13 days; in one colony the bees and mites died simultaneously. Though we did not observe robbing in our study, colonies with fewer than 200 bees still harbored mites that could have infested robber bees. All colonies attempted to rear worker-laid drones, though survival rates for the drones were low (3.0 ± 2.1% of worker-laid drone brood were estimated to reach adulthood). Colonies did, however, maintain adult drones until colony death, despite the experiment running from September through December (past the date of typical drone eviction). This work shows that declining colonies are a viable mechanism for horizontal mite transfer in both managed and wild colonies, with potential implications for the evolution of mite virulence.
Similar content being viewed by others
1 Introduction
The parasitic mite Varroa destructor has caused devastation in both managed and wild honey bee colonies around the world (Guzmán-Novoa et al. 2010; Martin et al. 2012; Mikheyev et al. 2015; Wilfert et al. 2016; reviewed in Traynor et al. 2020). Much research has focused on control of the parasite (Rosenkranz et al. 2010; Seeley and Griffin 2011; Al Toufailia et al. 2015), on the viruses it transmits (De Miranda and Genersch 2010; Wilfert et al. 2016; Benaets et al. 2017), on the longitudinal impacts on colony survival (Le Conte et al. 2007; Locke and Fries 2011; Seeley 2017; Locke 2016), and on honey bee traits that lead to mite resistance (Spivak 1996; Martin and Medina 2004; Ibrahim et al. 2007; Locke and Fries 2011; Loftus et al. 2016; Locke 2016), but less attention has been paid to the basic ecology of the mite itself (reviewed in Nazzi and Le Conte 2016).
Varroa mites spread between colonies by infesting a bee, which transports the mite to another colony. In apiaries, where colonies are densely packed, orientation errors lead to workers “drifting” between colonies, along with their mites (Jay 1966a, b; Pfeiffer and Crailsheim 1998; Seeley and Smith 2015; Smith and Loope 2016). Wild colonies, however, are widely spaced (> 1 km, though this depends on location; see Seeley 2007; 2019), so passive worker drift is not a viable mechanism for the horizontal transmission of mites. Nevertheless, wild colonies, even those living far from apiaries, are still infested with mites (Seeley 2007; Locke 2016). How wild colonies first become infested is unknown, but it has been speculated that the death of an infested colony may present an ideal opportunity for the resident mites in the dying colony to infest robber bees from foreign colonies, thereby escaping the dying colony and infesting a new host (Peck and Seeley 2019). This transmission route is viable in both managed colonies living in apiaries and unmanaged colonies living in the wild.
Surprisingly, however, we do not have a clear picture of how mite and bee populations covary within a terminally declining colony. This is important because the way in which an infected organism dies (whether killed by the disease or not) will influence whether and how widely it can transmit the infection (e.g., individuals infected with HIV have an extended asymptomatic period in which they can share specific bodily fluids containing high viral loads (Fauci 1993; Hollingsworth et al. 2008). To understand whether mites (and the viruses they transmit) will evolve higher or lower virulence, over time and in different settings, we must first understand any and all transmission routes between hosts (Ewald 1987, 2004; Fries and Camazine 2001). This includes potential transmission of mites during the process of colony decline and death, including death that is not explicitly caused by the mites.
While honey bees are a well-studied social insect (Butler 1623; Huber 1814; Frisch 1950; Lindauer 1961; Winston 1987; Seeley 1995, 2010), investigations into the patterns of colony growth and development are limited (Lee and Winston 1987; Pratt 1999; Rangel and Seeley 2012; Smith et al. 2016). This is because sociometric studies (i.e., the collection and analysis of the physical and numerical attributes of social insect colonies and their inhabitants; Tschinkel 1991, 2011) are labor-intensive and tedious, and such studies in honey bees tend to focus on their agricultural relevance (e.g., honey stores and colony size; Farrar 1937). While there has been work on initial colony founding, growth, and early reproduction (Otis 1973; Lee and Winston 1985, 1987; Pratt 1999; Rangel and Seeley 2012; Smith et al. 2014), there has only been one birth-until-death study in honey bees (Smith et al. 2016), and no study has explicitly focused on the sociometry of colony decline and death.
How a colony dies influences the potential pathways for mites to be exported to other colonies. A leading cause of death in wild honey bee colonies living in northern climates is winter starvation (Seeley 2017). Although mites can survive hours, or even days, without bees (De Guzman et al. 1993; Pettis et al. 2003), this type of colony death represents a dead end for the mites, since the nest would remain undiscovered by bees until the spring. When colonies perish at other times of year, however, conditions are more suitable for mites to spread. A common way for unmanaged honey bee colonies to die is during queen replacement, which occurs after a colony swarms, an unexpected queen death, or to replace an unhealthy/failing queen (supersedure, or matricide; Loope 2015). Once the colony rears a virgin queen, she must depart on her mating flight(s). If she fails to return (e.g., eaten by a bird), the colony will become “hopelessly queenless” —they have no source of fertilized eggs with which to rear workers or a replacement queen (Seeley 1985). The probability of failing to requeen, and, therefore, becoming hopelessly queenless, depends on local conditions (e.g., 4% in Ratnieks (1990) versus 12% in Smith (2018), both studies conducted in Ithaca, NY; 20–53% in Ali and Taha (2012), depending on the bee-eater migration). Workers in hopelessly queenless colonies continue to perform colony tasks (Naeger et al. 2013), though some will activate their ovaries and begin to lay unfertilized eggs, which develop into a final cohort of worker-laid drones (Page and Erickson 1988; Miller and Ratnieks 2001; Utaipanon et al. 2019). Without a source of new workers, however, the colony is doomed and will eventually perish. Surprisingly, beyond knowing that these colonies inevitably die, the sociometric dynamics of this type of colony death remain unexplored. This leaves us unable to evaluate the risk that mites will be exported during a colony’s process of decline and death.
In this study, we address two simultaneous knowledge gaps: (1) the dynamics of colony death after becoming hopelessly queenless, and (2) the potential for V. destructor mite transmission during this process of colony decline. Our goal was to observe the process of colony decline in hopelessly queenless colonies to answer basic ecological and epidemiological questions, such as: How long do hopelessly queenless colonies persist? How long do they pose a disease threat to uninfested colonies? What is the final cause of colony death? Who dies first—the bees or the mites? Are declining colonies invaded and robbed by foreign colonies? How successfully do these colonies rear worker-laid drones? And does this alter mite levels? To answer these questions, we tracked both colony metrics (number of workers and drones, area of worker brood, drone brood, honey stores) and mite metrics (number of adult mites and juvenile mites dying over time) in four colonies that were made hopelessly queenless until the death of the last bees and mites.
2 Methods
To observe the process of colony decline and its impact on V. destructor mite incidence, we established four hopelessly queenless honey bee colonies in observation hives and tracked the bees and mites until death. Note that this is not an investigation into how mite infestation kills a colony but, rather, how a colony’s death progresses and the effects of colony decline and death on the population of mites. These experiments were conducted in Apis mellifera colonies at Cornell University’s Liddell Field Station and the Dyce Lab for Honey Bee Studies in Ithaca, NY, USA (42°27.6′N, 76°26.7′W).
2.1 Observation hive set up
On day 0 (September 1, 2017), we established each colony into a 4-frame observation hive (each colony contains four “Langstroth-deep” style frames; frame dimensions: 43 × 20 cm; observation hive dimensions: 45 × 91 cm; as in Smith et al. 2017). Each colony contained the same nest contents: a frame of capped honey (top frame), two frames of mixed brood (eggs, uncapped larvae, capped larvae; middle two frames), a frame of an empty drone comb (25% of the total nest area, similar to unmanaged nests (Seeley and Morse 1976; Smith et al. 2016); bottom frame). Each colony was established with 2 frames fully covered with bees of mixed age (Imdorf et al. 1987), but no queen. On September 10, 2017, we opened the observation hives and removed all queen cells built by the workers, thereby eliminating any possibility of their rearing a replacement queen.
The bees and brood combs were sourced from research colonies (> 2.5 km away) that exclusively contained light-colored Cordovan bees, which allowed us to differentiate light-colored resident bees from dark-colored potential robbers (in Upstate New York, robbing is most likely to occur in late fall; Rangel and Seeley 2012; Peck and Seeley 2019). The source colonies were reared with high-mite levels for another study (Peck and Seeley 2019); each colony had an ample drone comb and was not treated for mites, so their mite levels were not kept artificially low by beekeeping management, such as acaricide treatments (each source colony had > 20 mites per 300 bees, measured using the sugar-roll method; Macedo et. al 2002). Hive entrances had distinct colored markings and were oriented in different directions to reduce drift between colonies. To enable mite surveys without disturbing the colonies, we modified the floor of the observation hives to insert a miniature sticky board (an oiled plastic board placed underneath 6-mm hardware cloth to capture fallen mites; installing a sticky board does not interfere with colony activities such as foraging or undertaking). This type of mite sampling was destructive, so we cannot say if fallen mites were alive or dead upon falling and whether any mites could have re-infested bees if they had not been killed by the oil.
2.2 Colony inspections
This study was deliberately performed using observation hives to allow for regular and repeated inspections, without disturbing internal colony processes (nest boxes would have to be opened to monitor the colony, which disrupts the colony, and can induce robbing). Every 48 h, we inspected colonies for the following: mite drop (dead mites collected on the sticky board; both adults and juveniles, judged by mite color: adults are reddish-brown, juveniles are white to cream-colored; Dietemann et al. 2013); presence of worker-laid eggs; and whether colonies were being robbed (non-Cordovan bees present in the observation hive), or had been robbed (robber bees tear open honey cells and leave characteristic debris behind, whereas resident bees carefully uncap honey cells). Hive debris fell through the screened bottom board, so large wax cap pieces indicative of robbing would have been easily detected.
A colony was considered alive for as long as adult workers or drones were alive in the observation hive. Every 7 days, at night, we censused: the population of workers using the Liebefeld method (Imdorf et al. 1987; Dainat et al. 2020), the population of drones by direct count (using a tally counter), and the contents of the nest (worker brood, drone brood, food stores) using a 4 × 4-cm grid square (55 grid squares per bee frame, methods as in Smith et al. (2016)). Toward the end of a colony’s life, we were able to make direct counts of both workers and drones living in the colony. Whenever a frame had fewer than 200 bees, we used a tally counter to directly count the number of individuals on that frame. These inspections continued until all mites and bees were dead, and all risk of robbing had ended for the season, on December 27, 2017. Note that this date is beyond the last bee’s death date (December 21) so that we could determine if any remaining mites were present (via mite drop and visual inspection). Putative cause of death was determined via visual inspection (e.g., starvation is easily detected because workers are found with their heads embedded in cells, and no honey/nectar remains in the colony; note that colonies are inspected every 48 h).
During these inspections, we also visually checked for the following honey bee diseases and pests (Hansen 1987): American foulbrood (Paenabacillus larvae), European foulbrood (Streptococcus pluton), chalkbrood (Ascosphaera apis), nosema-induced dysentery (Nosema apis), sacbrood virus, small hive beetles (Aethina tumida), and wax moths (Galleria mellonella).
2.3 Observation hive room
Observation hives flatten what would typically be a three-dimensional nest structure, which handicaps the bees’ ability to thermoregulate their nest. To account for this, hives were sandwiched between boards of foam insulation, and we partially regulated the temperature of the observation hive room using ventilation fans and space heaters such that it would not fall below 10 °C. A dwindling colony will eventually lack the bee population needed to properly thermoregulate its nest, and winter cold exerts a mortality pressure on all bee colonies in cool climates, so on December 20, 2017, we deactivated the heating system (three of the four colonies had already perished before this date). The last remaining colony was dead within 24 h. Clearly, environmental conditions influence how long a declining colony will last, but our temperature control helped us avoid any impacts from individual cold snaps on the longevity of the bees and/or mites due to their occupation of an essentially two-dimensional observation hive. While this does represent a semi-natural nest configuration (a two-dimensional observation hive versus a three-dimensional nest), using observation hives is essential for collecting high-resolution data on the number of bees and mites in the colony without disturbing the nest. This is an inherent trade-off in the experimental design.
2.4 Statistical analyses
Statistical analyses and figures were made in Python version 3.8, and R version 4.0.2, using the Pandas, Numpy, Scipy, Scikit-learn, and lme4 packages (McKinney 2011; Harris et al. 2020; Virtanen et al. 2020; Pedregosa et al. 2011; Bates et al. 2015; R Core Team 2020). To account for repeated mite counts per colony, we used a linear mixed effects model, comparing the number of workers in the colony (explanatory variable) and the number of adult mites (response variable), while controlling for colony ID (random factor). The best-fit model (null model versus model containing explanatory variables) was determined using likelihood ratio tests (Lewis et al. 2011) implemented in R using the anova() function. Residuals were checked for normality using Q-Q plots. After determining that worker number was a significant predictor of mite levels (with colony ID as a random slope or random intercept), we then performed a linear regression to calculate a slope and intercept across all data points, with number of workers as the independent variable, and number of adult mites as the dependent variable.
To calculate the number of adult drones that could have theoretically been reared into adults, we calculated the area under the drone brood curve (eggs, uncapped larvae, capped larvae; cm2 of a comb), multiplied by 2.73 to obtain the number of drone cells (6.5 mm wall-to-wall length), and divided by 24 days (the time from an egg to adult) (Seeley 2019). This is an upper limit for the number of adult drones that could have been reared based on the area of drone brood measured (i.e., it does not take into account a brood that could have been lost to cannibalization, hygienic removal, or death). To estimate the number of worker-laid drone eggs that successfully reached adulthood, we took the first date that worker-laid eggs were observed, added 24 days, and then noted the peak in drone population after that date. This is meant as a rough estimate, and an upper limit, as it does not account for drones that were installed with the colony, nor worker-laid drones that could have died immediately after eclosion. All data are reported as mean ± SD, unless otherwise noted.
3 Results: The dynamics of honey bee colony decline and death
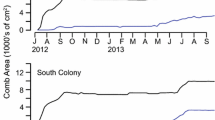
The first goal of this study was to observe colony dynamics during the process of decline and eventual death. Colonies survived for 86 ± 19 days (range: 61–111 days; Table I), with some colonies showing a heavy-tailed distribution (i.e., a high incidence of outliers; individuals that were particularly long-lived, see colony #1 and #4, Figure 1A, B). As long as honey stores remained (Figure 1G), declining colonies persisted even with only a few bees; colony #1, for example, had fewer than 200 workers from day 61 until its death 35 days later. On average, 50% of the bees were dead after 25 days; 95% of the bees were dead by day 74 (workers and drones combined; Figure 2).
Honey bee populations, V. destructor mite drops, and colony nest contents over time. Stars indicate endpoints for each metric (e.g., when worker number = 0), or a last observed instance (e.g., the last date juvenile mites were observed), with numbers noting the experimental date. The experiment began on September 1, 2017, experimental day 0. Rectangle in Di shows the region of the plot that is expanded in Dii. The black triangle in part E indicates the day queen cells were excised from all colonies (September 10, 2017), rendering them hopelessly queenless
Proportion of workers alive over time. Calculated relative to the peak number of workers in each colony (Figure 1A), the proportion of workers alive drops by 50%, on average, by day 25; by day 74, over 95% of workers have perished
In three of the four colonies (#1, 2, 3), the ultimate cause of death was starvation (colony #4 died with some honey stores, but insufficient bees to thermoregulate). Both workers and drones were present until colony death—the last date upon which the bees were observed alive included both workers and drones in three of the four colonies (#1, 2, 4). In only one colony did workers outlive the drones, by only 6 days (colony #3; Table I). Therefore, whereas workers in queenright colonies will evict and kill drones in the fall, the workers in these declining colonies maintained their drones through the fall and into the winter.
In all four colonies, the workers began laying eggs to rear drones—their last opportunity to achieve genetic success before death. Worker-laid eggs were detected after 32 ± 10 days (range: 19–42 days; Table I), and occupied up to 528 ± 307 cm2 of the nest (range, 96–960 cm2; calculated as the maximum drone-brood area per colony on a given date; note that the comb on one side of one bee frame measures 44 × 20 cm = 880 cm2). Calculating the area underneath the drone-brood curve (Figure 1F), one would predict that thousands of drones had been reared in these four colonies (1586 ± 1207 drones; range, 317–3562; Table I, see methods). However, the number of worker-laid drones that successfully reached adulthood ranged from 0 to 107 (Table I). Evidently, although workers in a declining colony are capable of laying a large area of a drone brood (over 1/8th of the comb surface in this study), their ability to rear the drone eggs to adulthood is 1 to 3 orders of magnitude lower (i.e., the difference between drone eggs laid and adult drones reared).
During our inspections, other than Varroa, we did not observe evidence of other honey bee diseases or pests (American foulbrood, European foulbrood, chalkbrood, Nosema apis, sacbrood, small hive beetles, wax moths).
4 Results: V. destructor mite incidence during honey bee colony decline
The second goal of this study was to determine how V. destructor mite populations change during the process of colony decline and death. In these declining colonies, adult workers and drones were available to host parasitic mites for between 61 and 111 days (Figure 1A, Table I). The number of adult mites that were collected was positively correlated with the number of workers in the colony (linear regression: F1,41 = 37.63, intercept = 3.57, slope = 0.016, R2 = 0.467, P < 0.001; LMER controlling for colony ID: P < 0.001; Figure 3). However, even when colonies had fewer than 200 workers, adult mites were still found in all 4 colonies (Figures 1A, C, and 3ii).
Adult mite drops are correlated with the number of workers in the colony. At left, part i, all data are shown, with a dotted line showing the results of linear regression (F1,41 = 37.63, intercept = 3.57, slope = 0.016, R2 = 0.467, P < 0.001). The rectangle outlines small-colony sizes, which are plotted at right, part ii. Note that, even when there are fewer than 200 workers present in the colony, adult mites are still detected
Given that mites depend upon bees for their survival, who perishes first: the bees or the mites? In colony #1, the bees outlived the mites—the last observed adult mite was found 13 days before the last worker bee died (Table I). In colony #4, the bees and the mites died simultaneously (i.e., they perished on the same day). In two of the four colonies, however, the mites outlived the bees, by up to 48 h—in both colonies #2 and #3, the last observed adult mite was found dead 2 days after the last worker bee had died (Table I). Mite inspections occurred every 2 days, even after the last bee died, so this represents an upper limit for the longevity of the mites once there are no adult workers left to parasitize. However, this does indicate that, during colony decline, adult mites may be present for as long as adult bees are still alive and for at least 2 days afterward.
To reproduce, mites require cells of capped brood. Without a queen laying fertilized eggs, there was no longer a source of worker brood, and, by day 21, all the worker brood that was installed with the colony had eclosed (Figure 1E). The workers in the colony, however, began laying unfertilized eggs as early as 19 days post-installation (Table I), which provided a potential source of capped drone brood, which could be used for mite reproduction (Figure 1F). Light-colored putatively juvenile mites can be used as a proxy for mite reproduction (Martin 1995), and we did find juvenile mites in all 4 colonies, even after all the worker brood had eclosed. In only 1 of the 4 colonies, however, did we find more than an occasional juvenile mite (Colony #2; Table I; Figure 1D). In this colony (#2), we collected a total of 53 juvenile mites after day 25, a tenfold increase as compared to the other three colonies (Table I). The workers in colony #2 also exhibited the highest reproductive investment of the four colonies: (1) they were the first to begin laying unfertilized eggs, (2) they had the greatest area of drone brood, and (3) they reared the most drones to adulthood (Table I; Figure 1B, F). Therefore, worker reproduction in declining colonies provided a final opportunity for mite reproduction, but worker reproduction was not necessary for mites to be maintained until colony death; colony #3 reared 0 drones to adulthood, and yet the mites still outlived the bees in this colony.
Finally, what were the prospects for the mites in these declining colonies to be transmitted to foreign colonies? The cause of death for three of the four colonies was starvation, and none of the colonies were robbed by foreign bees before they died. Therefore, at least in our experimental setup, the mites that lived in these declining colonies perished with the bees.
5 Discussion
The goal of this study was to track the dynamics of honey bee colony decline and death, as well as how V. destructor mite population changes during this process. Despite having no queen to produce new workers, these doomed colonies persisted for 2–3 months; their eventual cause of death was a combination of starvation and exposure to cold temperatures. This timeline is longer than what would be expected for “summer” bees, which live 4–6 weeks but shorter than what would be expected for “winter” bees, which live up to 8 months (Fukuda and Sekiguchi 1966; Seeley 1985; Mattila et al. 2001). Of course, longevity depends on multiple factors, both internal and external to the colony. Mites were present in the colonies for as long as the workers were alive, and even up to 48 h after. Therefore, declining colonies theoretically remain “infectious” to their neighbors throughout the process of colony death and can serve as a source of mite transmission should any foreign workers discover and rob the declining colony’s honey stores (Frey et al. 2011; Frey and Rosenkranz 2014; Peck and Seeley 2019). While we did not observe robbing in our experiment, it is common in apiaries where colonies are crowded together (Free 1954; Root 2007) and even among colonies spaced further apart (Peck and Seeley 2019; Seeley 2019). These experiments were performed in colonies that died due to queenlessness, but similar experiments could be done in colonies that die of an excessive mite infestation, which could change both the process of colony decline and the potential for mite transmission.
All four colonies attempted to rear worker-laid drones—worker-laid eggs appeared after 32 ± 10 days, which aligns with previous work (Miller and Ratnieks 2001). Their success in rearing drone eggs to adulthood, however, was limited. Workers in queenless colonies do continue to perform colony tasks (e.g., foraging, defense: Naeger et al. 2013), but the mismatch between drone eggs and drone adults demonstrates that queenless colonies are not as capable at rearing drones as queenright colonies, likely due to multiple coinciding factors in a dying colony (cannibalism, hygienic removal of dead brood, worker-worker competition, etc.). The area of drone brood laid by the workers could theoretically have led to 1586 ± 1207 adult drones, but, instead, only 41 ± 40 drones were reared to adulthood (per-colony values in Table I). This is a 3.0 ± 2.1% survival rate for worker-laid drone eggs in a hopelessly queenless colony. Drone eggs in a queenright colony have a survival rate of 55.8% (Fukuda and Ohtani 1977), but those are queen-laid eggs, and workers preferentially consume worker-laid eggs over queen-laid eggs (Loope et al. 2013). While 3.0% survival of worker-laid eggs in queenless colonies may seem low, this is higher than the survival of worker-laid eggs in queenright colonies—only 0.12% reach drone adulthood due to worker policing (Visscher 1989). Nonetheless, these hopelessly queenless colonies are not as successful at rearing drone eggs to adulthood as they are when queenright—a potential consequence of competition between simultaneously reproductive workers in their final effort for individual genetic success.
This final bout of worker reproduction provided an opportunity for mites to also reproduce. We did observe juvenile mites in the colonies (an indicator of mite reproduction), but we did not observe a drastic increase in adult mite counts during worker reproduction (Figure 1C). Therefore, while declining colonies do provide an opportunity for mites to continue reproduction and, perhaps, even grow their numbers before the colony’s inevitable death, we did not observe explosive mite growth during the process of colony decline and death (perhaps because these colonies only reared a few drones to adulthood). Given that workers in hopelessly queenless colonies are not particularly adept at rearing their drone brood to adulthood (Table I), it may be a risky evolutionary strategy for mites to reproduce in the drone cells of a declining colony, as they could be trapped inside drone cells that never hatch (in three of the four colonies, there was still capped drone brood present when the last bee had perished; colonies #1, 2, and 3 in Figure 1F). If the colony gets robbed while the mites are within a cell, they may miss their only opportunity to be transported to a new host colony.
Workers typically expel adult drones from the colony in the fall or when food reserves are severely limited (Free 1957; Free and Williams 1975; Cicciarelli 2013; Smith et al. 2016), but workers in these hopelessly queenless colonies maintained their adult drones throughout the process of colony decline and death, despite frigid outdoor temperatures. While it is unlikely that virgin queens were available for drones to mate with in Ithaca NY in December, these drones represent the worker’s only possibility, however small, to achieve genetic success. This is another example of how worker behavior is dependent on the state of the colony (e.g., comb building changes with the queen’s mating status (Smith 2018); the presence of larvae increases the probability of individual fanning behavior (Cook et al. 2016); foraging signals change depending on the availability of storage space (Kietzman and Visscher 2021)).
We performed this experiment in September, when robbing occurs in the area (Peck and Seeley 2019), but also to align with the 2nd swarming peak, when colonies rear replacement queens (in Upstate NY, swarming primarily occurs in May/June, but there is a second peak in August/September; Fell et al. 1977; Smith et al. 2016). Therefore, the time of year was chosen to mimic conditions when colonies could become hopelessly queenless and also be robbed as the colony declines. Of course, a colony’s queen can fail at any time of year, so the scope of this study is limited by the time during which it was conducted, and the location—a colony that becomes hopelessly queenless in Ithaca NY in May faces a different set of environmental challenges than does one in September. As noted in the methods, we did heat the room in which the observation hives were kept to mitigate the additional stress on the colonies of having to thermoregulate a 2-dimensional observation hive. Three of the four colonies perished before we turned off the heaters on December 20, 2017, and the final, dwindling colony (#4) was dead within 24 h. While heating the room likely extended the life of these colonies in the fall, it does show that hopelessly queenless colonies are capable of surviving without replacement workers for 2–3 months and, perhaps, even longer had there been nectar available in the environment. Of course, every sociometric study is inherently tied to local conditions (e.g., location, season, initial colony state, experimental design); how these conditions influence the longevity of hopelessly queenless colonies could be addressed by future work.
The results of this study have implications for how mites are transmitted in unmanaged colonies and within apiaries: (1) The process of colony decline and death can occur over months, with a long-tailed distribution of long-lived workers and drones (Figures 1A, B and 2); (2) declining colonies remain a potential source of mites until the last bee perishes (Figures 1C and 3); (3) worker reproduction may facilitate a last bout of mite reproduction, though the scale depends on conditions in the colony (Figure 1C, D, and F). In apiaries, workers frequently drift between colonies (over 40% in some conditions; Free 1958; Jay 1966a, b; Pfeiffer and Crailsheim 1998), and so a hopelessly queenless colony may not change the amount of mite mixing that already occurs between managed colonies. Mites can reproduce in the worker-laid brood, but we did not observe a spike in mite population as workers began to reproduce. Therefore, at least in our experimental setup, hopelessly queenless colonies do not appear to supercharge mite reproduction, despite the rapid shift to drone rearing.
In natural settings, colonies are widely spaced, and so substantial drift between colonies does not occur (Seeley and Smith 2015; Seeley 2019). However, wild colonies still harbor mites, and a long-standing question has been how mites are capable of infesting and spreading between unmanaged colonies living in the wild. Some proposed mechanisms, such as a mite falling from a bee while at a flower and then infesting a new bee that visits that same flower, are plausible (Peck et al. 2016) but likely rare and could not explain the ubiquity of V. destructor mites in wild colonies (Seeley 2007). By tracking the process of colony decline and death, along with the population dynamics of their mites, we show that V. destructor can persist with its host throughout the process of decline and death (and even hours beyond the colony’s death). Given the long-tailed distribution of bees left in the declining colony, this makes mite dispersal via robbing a plausible mechanism for how wild colonies become infested with mites, though we did not observe robbing in our experimental setup. This putative mechanism provides a route for the horizontal transmission of mites and mite-associated viruses, which favors higher virulence than vertical transmission (i.e., mother to offspring, vertical transmission: mites are present in both the mother-swarm and daughter-nest colonies). For a review on virulence in horizontal versus vertical transmission, see Fries and Camazine (2001). A long and slow death provides the greatest opportunity for declining colonies to be discovered and robbed and, possibly, to spread their mites and other pathogens to colonies in the area. Our work shows that, as long as a single resident bee remains in a declining colony, there is a possibility for mites to infest intruders and, thereby, be carried to a new hospitable host.
Data availability
The data used in this study are available at AUrora, the Auburn University online digital repository: http://dx.doi.org/10.35099/aurora-547.
Code availability
Not applicable.
References
Ali MA, Taha MA (2012) Bee-eating birds (Coraciiformes: Meropidae) reduce virgin honey bee queen survival during mating flights and foraging activity of honey bees (Apis mellifera L.). Int J Sci Eng Res 3(6):1–8
Bates D, Mächler M, Bolker BM, and Walker SC (2015) “Fitting linear mixed-effects models using Lme4.” J Stat Softw 67 (1): 1–48. https://doi.org/10.18637/jss.v067.i01
Benaets K, Geystelen AV, Cardoen D, Smet L, Graaf DC, Schoofs L, Larmuseau MHD, Brettell LE, Martin SJ, Wenseleers T (2017) Covert deformed wing virus infections have long-term deleterious effects on honeybee foraging and survival. Proc R Soc B. https://doi.org/10.1098/rspb.2016.2149
Butler C (1623) The feminine monarchie: or the historie of bees. Oxford, UK: Joseph Barnes
Cicciarelli RP (2013) Drone eviction in honey bees (Apis mellifera). Cornell University
Cook CN, Durzi KJS, Breed MD (2016) Larvae influence thermoregulatory fanning behavior in honeybees (Apis mellifera L.). Insectes Soc 63(2):271–278. https://doi.org/10.1007/s00040-016-0463-5
Dainat B, Dietemann V, Imdorf A, Charrière JD (2020) A scientific note on the ‘Liebefeld Method’ to estimate honey bee colony strength: its history, use, and translation. Apidologie 51(3):422–427. https://doi.org/10.1007/s13592-019-00728-2
Dietemann V, Francesco N, Martin SJ, Anderson DL, Locke B, Delaplane KS, Wauquiez Q, et al. (2013) Standard methods for Varroa research. J Apic R 52 (1). https://doi.org/10.3896/IBRA.1.52.1.09
Ewald PW (1987) Transmission modes and evolution of the parasitism-mutualism continuum. Ann N Y Acad Sci 503(1):295–306. https://doi.org/10.1111/j.1749-6632.1987.tb40616.x
Ewald PW (2004) Evolution of virulence. Infect Dis Clin North Am 18(1):1–15. https://doi.org/10.1016/S0891-5520(03)00099-0
Farrar CL (1937) The influence of colony populations on honey production. J Agric Res 54(12):945–954
Fauci AS (1993) Immunopathogenesis of HIV infection. J Acquir Immune Defic Syndr 6(6):655–662
Fell RD, Ambrose JT, Burgett DM, Dejong D, Morse RA, Seeley TD (1977) Seasonal cycle of swarming in honeybees. J Apic Res 16(4):170–173
Free JB (1954) The behaviour of robber honeybees. Behaviour 7(2/3):233–240
Free JB (1958) The drifting of honey-bees. J Agric Sci 51 (03): 294–306. http://journals.cambridge.org/abstract_S0021859600035103
Free JB (1957) The food of adult drone honeybees (Apis mellifera). Br J Anim Behav 1:14–18. https://doi.org/10.1016/S0950-5601(57)80038-0
Free JB, Williams IH (1975) Factors determining the rearing and rejection of drones by the honeybee colony. Anim Behav 23:650–675
Frey E, Rosenkranz P (2014) Autumn invasion rates of Varroa destructor (Mesostigmata: Varroidae) into honey bee (Hymenoptera: Apidae) colonies and the resulting increase in mite populations. J Econ Entomol 107(2):508–515
Frey E, Schnell H, Rosenkranz P (2011) Invasion of Varroa destructor mites into mite-free honey bee colonies under the controlled conditions of a military training area. J Apic Res 50(2):138–144
Fries I, Camazine S (2001) Implications of horizontal and vertical pathogen transmission for honey bee epidemiology. Apidologie 32:199–214
Frisch Kv (1950) Bees: their vision, chemical senses, and language. Oxford University Press 1:157
Fukuda H, Ohtani T (1977) Survival and life span of drone honeybees. Res Popul Ecol, 51–68
Fukuda H, Sekiguchi K (1966) Seasonal change of the honeybee worker longevity in Sapporo, North Japan, with notes on some factors affecting the life-span. Jpn J Ecol 16(5):206–12
Guzmán-Novoa E, Eccles L, Calvete Y, McGowan J, Kelly PG, Correa-Benítez A (2010) Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie 41(4):443–450. https://doi.org/10.1051/apido/2009076
Guzman LI, Rinderer TE, Beaman LD (1993) Survival of Varroa jacobsoni Oud. (Acari: Varroidae) away from its living host Apis mellifera L. Exp Appl Acarol 17(4):283–290. https://doi.org/10.1007/BF02337278
Hansen H (1987) Honey Bee Brood Diseases. Wicwas Press, Ithaca, NY
Harris CR, Millman KJ, Walt SJ, Gommers R, Virtanen P, Cournapeau D, Wieser E et al (2020) Array programming with NumPy. Nature 585:357–362. https://doi.org/10.1038/s41586-020-2649-2
Hollingsworth TD, Anderson RM, Fraser C (2008) HIV-1 transmission, by stage of infection. J Infect Dis 198(5):687–693. https://doi.org/10.1086/590501
Huber F (1814) New observations upon bees. Hamilton, IL: American Bee Journal
Ibrahim A, Reuter GS, Spivak M (2007) Field trial of honey bee colonies bred for mechanisms of resistance against Varroa destructor. Apidologie 38(1):67–76. https://doi.org/10.1051/apido:2006065
Imdorf A, Buehlmann G, Gerig L, Kilchenmann V, Wille H (1987) Überprüfung Der Schätzmethode Zur Ermittlung Der Brutfläche Und Der Anzahl Arbeiterinnen in Freifliegenden Bienenvölkern. Apidologie 18(2):137–146
Jay SC (1966a) Drifting of honeybees in commercial apiaries III: effect of apiary layout. J Apic Res 5(3):137–148
Jay SC (1966b) Drifting of honeybees in commercial apiaries II: effect of various factors when hives are arranged in rows. J Apic Res 5(2):103–112
Kietzman PM, Visscher PK (2021) The influence of available comb storage space on the performance of honey bee communication signals that regulate foraging. Apidologie 52(1):133–140. https://doi.org/10.1007/s13592-020-00803-z
Le Conte Y, De Vaublanc G, Crauser D, Jeanne F, Rousselle JC, Bécard JM (2007) Honey bee colonies that have survived Varroa destructor. Apidologie 38(6):566–72. https://doi.org/10.1051/apido:2007040
Lee PC, Winston ML (1985) The effect of swarm size and date of issue on comb construction in newly founded colonies of honeybees (Apis mellifera L.). Can J Zool 63(3):524–527. https://doi.org/10.1139/z85-077
Lee PC, Winston ML (1987) Effects of reproductive timing and colony size on the survival, offspring colony size and drone production in the honey bee (Apis mellifera). Ecological Entomology 12(2):187–195. https://doi.org/10.1111/j.1365-2311.1987.tb00997.x
Lewis F, Butler A, Gilbert L (2011) A unified approach to model selection using the likelihood ratio test. Methods Ecol Evol 2(2):155–62. https://doi.org/10.1111/j.2041-210X.2010.00063.x
Lindauer M (1961) Communication among social bees. Harvard University Press, Cambridge, MA
Locke B (2016) Natural Varroa mite-surviving Apis mellifera honeybee populations. Apidologie 47(3):467–482. https://doi.org/10.1007/s13592-015-0412-8
Locke B, Fries I (2011) Characteristics of honey bee colonies (Apis mellifera) in Sweden surviving Varroa destructor infestation. Apidologie 42(4):533–542. https://doi.org/10.1007/s13592-011-0029-5
Loftus JC, Smith ML, Seeley TD (2016) How honey bee colonies survive in the wild: testing the importance of small nests and frequent swarming. PLoS ONE 11 (3). https://doi.org/10.1371/journal.pone.0150362
Loope KJ (2015) Queen killing is linked to high worker-worker relatedness in a social wasp. Curr Biol 25(22):2976–2979. https://doi.org/10.1016/j.cub.2015.09.064
Loope, KJ, Seeley TD, Mattila HR (2013) No facultative worker policing in the honey bee (Apis mellifera L.). Die Naturwissenschaften, 6–10. https://doi.org/10.1007/s00114-013-1025-6
Macedo PA, Wu J, Ellis MD (2002) Using inert dusts to detect and assess Varroa infestations in honey bee colonies. J Apic Res 41(1–2):3–7. https://doi.org/10.1080/00218839.2002.11101062
Martin SJ (1995) Reproduction of Varroa jacobsoni in cells of Apis mellifera containing one or more mother mites and the distribution of these cells. J Apic Res 34(4):187–196. https://doi.org/10.1080/00218839.1995.11100904
Martin SJ, Highfield AC, Brettell L, Villalobos EM, Budge GE, Powell M, Nikaido S, Schroeder DC (2012) Global honey bee viral landscape altered by a parasitic mite. Science 6086:1304–1306. https://doi.org/10.1126/science.1220941
Martin SJ, Medina L (2004) Africanized honeybees have unique tolerance to Varroa mites. Trends Parasitol 20(3):112–114. https://doi.org/10.1016/j.pt.2003.12.005
Mattila HR, Harris JL, Otis GW (2001) Timing of production of winter bees in honey bee (Apis Mellifera) colonies. Insectes Soc 48(2):88–93. https://doi.org/10.1007/PL00001764
McKinney W (2011) Pandas: a foundational Python library for data analysis and statistics. Python for high performance and scientific computing 14.9: 1-9.
Mikheyev AS, Tin MMY, Arora J, Seeley TD (2015) Museum samples reveal rapid evolution by wild honey bees exposed to a novel parasite. Nat Commun 6:1–8. https://doi.org/10.1038/ncomms8991
Miller DG III, Ratnieks FLW (2001) The timing of worker reproduction and breakdown of policing behaviour in queenless honey bee (Apis Mellifera L.) societies. Insect Soc 48:178–84. https://doi.org/10.1007/PL00001762
Miranda JR, Genersch E (2010) Deformed wing virus. J Invertebr Pathol 103:S48–61. https://doi.org/10.1016/j.jip.2009.06.012
Naeger NL, Peso M, Even N, Barron AB, Robinson GE (2013) Altruistic behavior by egg-laying worker honeybees. Curr Biol 23(16):1574–1578
Nazzi F, Le Conte Y (2016) Ecology of Varroa destructor, the major ectoparasite of the Western honey bee, Apis mellifera. Annu Rev Entomol 61:417–432
Otis GW (1973) The swarming biology and population dynamics of the Africanized honey bee. University of Kansas, 1980
Page RE Jr, Erickson EH Jr (1988) Reproduction by worker honey bees (Apis Melifera L.). Behav Ecol Sociobiol 23:117–26. https://doi.org/10.1007/BF00299895
Peck DT, Smith ML, Seeley TD (2016) Varroa destructor mites can nimbly climb from flowers onto foraging honey bees. PLoS ONE 11 (12): e0167798. https://doi.org/10.1371/journal.pone.0167798
Peck DT, Seeley TD (2019) Mite bombs or robber lures? The roles of drifting and robbing in Varroa destructor transmission from collapsing honey bee colonies to their neighbors. PLoS ONE 14(6):1–14. https://doi.org/10.1371/journal.pone.0218392
Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M et al (2011) Scikit-learn: machine learning in Python. J Mach Learn Res 12:2825–2830
Pettis JS, Ochoa R, Orr J (2003) Interception of a live Varroa mite on imported cut flowers in the United States. Int J Acarology 29(3):291–292. https://doi.org/10.1080/01647950308684342
Pfeiffer KJ, Crailsheim K (1998) Drifting of honeybees. Insectes Soc 45(2):151–167. https://doi.org/10.1007/s000400050076
Pratt SC (1999) Optimal timing of comb construction by honeybee (Apis mellifera) colonies: a dynamic programming model and experimental tests. Behav Ecol Sociobiol 46(1):30–42. https://doi.org/10.1007/s002650050589
R Core Team (2020) “R: a language and environment for statistical computing.” Vienna, Austria. http://www.r-project.org
Rangel J, Seeley TD (2012) Colony fissioning in honey bees: size and significance of the swarm fraction. Insectes Soc 59(4):453–462. https://doi.org/10.1007/s00040-012-0239-5
Ratnieks FLW (1990) The evolution of polyandry by queens in social hymenoptera: the significance of the timing of removal of diploid males. Behav Ecol Sociobiol 26(5):343–348. https://doi.org/10.1007/BF00171100
Root AI (2007) The ABC & XYZ of bee culture. Edited by H. Shimanuki, K Flottum, A Harman, and S Garceau. 41st ed. Medina, OH: A.I. Root Co
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Invertebr Pathol 103:S96–119. https://doi.org/10.1016/j.jip.2009.07.016
Seeley TD (1985) Honeybee ecology: a study of adaptation in social life. Princeton University Press, Princeton, New Jersey
Seeley TD (2010) Honeybee democracy. Princeton University Press, Princeton, New Jersey
Seeley TD (2007) Honey bees of the Arnot Forest: a population of feral colonies persisting with Varroa destructor in the Northeastern United States. Apidologie 38:19–29. https://doi.org/10.1051/apido:2006055
Seeley TD (1995) The wisdom of the hive: the social physiology of honey bee colonies. Harvard University Press. Cambridge, MA: Harvard University Press. https://doi.org/10.1016/j.desal.2010.03.003
Seeley TD (2017) Life-history traits of wild honey bee colonies living in forests around Ithaca, NY, USA. Apidologie 48(6):743–754. https://doi.org/10.1007/s13592-017-0519-1
Seeley TD (2019) The lives of bees: the untold story of the honey bee in the wild. Princeton University Press, Princeton, New Jersey
Seeley TD, Griffin SR (2011) Small-cell comb does not control Varroa mites in colonies of honeybees of European origin. Apidologie 42(4):526–532. https://doi.org/10.1007/s13592-011-0054-4
Seeley TD, Morse RA (1976) The nest of the honey bee (Apis Mellifera L.). Insectes Soc 23(4): 495–512. https://doi.org/10.1007/BF02223477
Seeley TD, Smith ML (2015) Crowding honeybee colonies in apiaries can increase their vulnerability to the deadly ectoparasite Varroa destructor. Apidologie 46(6):716–727. https://doi.org/10.1007/s13592-015-0361-2
Smith ML, Ostwald MM, Seeley TD (2016) Honey bee sociometry: tracking honey bee colonies and their nest contents from colony founding until death. Insectes Soc 63(4):553–563. https://doi.org/10.1007/s00040-016-0499-6
Smith ML, Loope KJ (2016) Caught in an evolutionary trap: worker honey bees that have drifted into foreign colonies do not invest in ovary activation. Insectes Soc 63(1):61–65. https://doi.org/10.1007/s00040-015-0434-2
Smith ML, Ostwald MM, Loftus JC, Seeley TD (2014) A critical number of workers in a honeybee colony triggers investment in reproduction. Naturwissenschaften 101(10):783–790. https://doi.org/10.1007/s00114-014-1215-x
Smith ML (2018) Queenless honey bees build infrastructure for direct reproduction until their new queen proves her worth. Evolution 72(12):2810–2817. https://doi.org/10.1111/evo.13628
Smith ML, Koenig PA, Peters JM (2017) The cues of colony size: how honey bees sense that their colony is large enough to begin to invest in reproduction. J Exp Biol 220(9):1597–1605. https://doi.org/10.1242/jeb.150342
Spivak M (1996) Honey bee hygienic behavior and defense against Varroa jacobsoni. Apidologie 27:245–260
Toufailia HA, Scandian L, Ratnieks FLW (2015) Towards integrated control of Varroa: 2) comparing application methods and doses of oxalic acid on the mortality of phoretic Varroa destructor mites and their honey bee hosts. J Apic Res 54(2):108–120. https://doi.org/10.1080/00218839.2015.1106777
Traynor KS, Mondet F, de Miranda JR, Techer M, Kowallik V, Oddie MAY, Chantawannakul P, McAfee A (2020) Varroa destructor: a complex parasite, crippling honey bees worldwide. Trends Parasitol 36(7):592–606. https://doi.org/10.1016/j.pt.2020.04.004
Tschinkel WR (1991) Insect sociometry, a field in search of data. Insectes Soc 38(1):77–82. https://doi.org/10.1007/BF01242715
Tschinkel WR (2011) Back to basics: sociometry and sociogenesis of ant societies (Hymenoptera: Formicidae). Myrmecological News 14:49–54
Utaipanon P, Holmes MJ, Oldroyd BP (2019) Queenless colonies contribute to the male breeding population at honey bee drone congregation areas. Insectes Soc 66(4):593–99. https://doi.org/10.1007/s00040-019-00720-0
Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, Burovski E et al (2020) SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods 17:261–272. https://doi.org/10.1038/s41592-019-0686-2
Visscher PK (1989) A quantitative study of worker reproduction in honey bee colonies. Behav Ecol Sociobiol 25(4):247–254
Wilfert L, Long G, Leggett HC, Schmid-Hempel P, Butlin R, Martin SJ, Boots M (2016) Deformed wing virus is a recent global epidemic in honeybees driven by Varroa mites. Science 6273:594–597. https://doi.org/10.1126/science.aac9976
Winston ML (1987) Biology of the honey bee. Harvard University Press, Cambridge, MA
Acknowledgements
We thank Chris Adam for help with setting up colonies and beekeeping expertise, Scott McArt for providing space to house the observation hives, and Tom Seeley for providing access to source colonies, research materials, and dedicated mentorship.
Funding
This research was supported by the NSF GRFP (DGE – 1144153 to DTP and MLS), and the NSF DDIG (1600775; MLS).
Author information
Authors and Affiliations
Contributions
MLS and DTP conceptualized the research and collected the data. MLS performed the data analysis and data visualization, and wrote the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Manuscript editor Yves Le Conte.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Smith, M.L., Peck, D.T. Dynamics of honey bee colony death and its implications for Varroa destructor mite transmission using observation hives. Apidologie 54, 13 (2023). https://doi.org/10.1007/s13592-023-00991-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-023-00991-4