Abstract
We describe a system for the in situ collection of volatiles from bees enclosed on a standard Langstroth frame face. The system includes an observation frame consisting of a glass plate and an aluminum frame that encloses a single frame face. A push–pull airflow system and an in-line volatile collection filter allow for air exchange and headspace volatile capture. This system can provide insight into colony chemical communication. The emissions of four compounds (2-heptanone, methyl benzoate, decanal, and 3-carene) associated with adult bees or colony materials remained steady or increased slightly in repeated collections from frames with maturing larvae. The emissions of the larval food component octanoic acid reflected changes in food consumption patterns by differently aged larvae. The production of the primer pheromone E-β-ocimene was greatest in comb containing young larvae and recently capped brood, but was lower on comb with capping larvae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Honeybee colonies emit a complex of volatile (airborne) compounds, specific subsets of which come from individuals, hive materials, and stores within the colony (Blum and Fales 1988; Winston 1987). Together with non-volatile (contact) and semivolatile colony chemicals, colony volatiles function as pheromones and kairomones to signal various biological states to receiving individuals both within and outside of the colony (Pankiw 2004; Alaux et al. 2010). Volatiles serve key roles as pheromone cues in defense, alarm, orientation, hygiene, foraging, and brood-rearing decisions (Breed et al. 2004; Hunt 2007; Vallet et al. 1991; Stout and Goulson 2001; Slessor et al. 2005a, b; Swanson et al. 2009; Maisonnasse et al. 2010). In turn, colony volatiles are exploited by other honeybee colonies and natural enemies (Nazzi et al. 2009; Torto et al. 2007). Volatiles could potentially also serve as chemical markers of stress such as poor nutrition, disease, and colony disturbance (Dussaubat et al. 2010). Aside from providing insights into potential semiochemicals, volatile markers could provide a non-intrusive means of assessing honeybee colony health.
One area of interest is the volatiles associated with honeybee larvae and how these are perceived and responded to by adult bees. As completely dependent members of the colony, honeybee larvae signal their biological state and needs to attendant worker bees by a variety of chemical cues (Haydak 1970; Free and Winder 1983; Huang and Otis 1991). Brood-associated volatiles are suspected in a variety of larval–worker interactions including hygienic behavior, brood care, feeding, and cell invasion by the Varroa mite Varroa destructor (Garrido and Rosenkranz 2004; Gramacho and Spivak 2003; Nazzi et al. 2004, 2009; Palacio et al. 2010; Swanson et al. 2009), although few of the specific cues responsible for these activities have been identified. Recently, the novel volatile primer pheromone E-β-ocimene was found to affect worker foraging behavior and suppress ovary development (Maisonnasse et al. 2009, 2010).
Both worker bees and colony parasites specifically respond to the chemical signals from these immobile larvae in their cells (Gramacho and Spivak 2003; Nazzi et al. 2004, 2009). In bees, like other insects, most interactions mediated by volatile semiochemicals depend on the effective volatile concentration present in the airspace (Slessor et al. 2005a, b). Most volatiles examined in honeybees vary quantitatively between individuals (Blum and Fales 1988). Differences in volatile compounds are rarely absolute in honeybees, except between castes and sex (Breed et al. 2004). Honeybees give off small amounts of volatiles even when not engaging in full volatile release (i.e., alarm pheromone concentrations in undisturbed bees; Torto et al. 2007). To add to the complexity, most honeybee volatiles are released in complex mixtures with synergistic effects (Boch et al. 1970; Pickett et al. 1982). Therefore, techniques for the analysis of volatile emissions must provide reliable and accurate means for quantification under conditions that are as natural as possible.
Unfortunately, accurate representative collections of volatiles from honeybee larvae are difficult to obtain using conventional techniques. Most volatile sampling techniques involve the removal of the larvae from the cell environment. Solvent extractions of bees have long been used to identify and characterize both volatile and non-volatile semiochemicals (Blum and Fales 1988), including brood ester pheromones (Le Conte et al. 1989, 1990), alarm pheromones (Barbier and Lederer 1960; Shearer and Boch 1962; Collins and Blum 1983; Blum and Fales 1988), Nasanov pheromones (Shearer and Boch 1965), and various queen pheromone components (Slessor et al. 1988; Keeling et al. 2003; see Winston 1987). However, solvent extractions of volatiles from larvae do not yield information about biologically meaningful volatile emission rates, but only the chemical contents of the tissues. Differences in biosynthesis or chemical storage turnover rates can lead to biased estimates of released amounts. In addition, solvent washes contain many extraneous, non-volatile compounds that are not components of odors (Heath and Manukian 1994).
Volatile collections from isolated individuals allow for accurate estimations of volatile emission rates, but may create stress artifacts (Torto et al. 2007). Alternatively, researchers have used open-air volatile collection techniques to sample odors from natural interactions as these events occur in the colony environment (Thom et al. 2007). But open-air volatile collections are rarely specific because of the surrounding heterogeneity of the colony environment. Also, compared to closed sampling systems, open-air sampling of whole colony airspace reduces the sensitivity and accuracy needed for the quantification of volatiles (Heath and Manukian 1994).
In this paper, we describe a novel method for the in situ collection of volatiles from relatively undisturbed active brood comb. We introduce the observation frame, a glass and aluminum frame used to fully enclose the bees and comb headspace of a single frame face. We also describe a closed push–pull airflow system used in combination with adsorbent in-line filters to trap and quantify headspace volatiles. The use of this push–pull airflow system with the observation frame in an incubator allows us to minimize disturbance and optimize sensitivity.
As an example of volatile collections from undisturbed larvae, we compared volatiles emitted by differently aged brood and their adult caretakers in the native comb environment. We selected six volatile compounds for analysis based on their known occurrence and utility in honeybee colonies. Some of these volatiles have known semiochemical roles in honeybee colonies. Four of the compounds have been reported from adult bees or colony materials, while two have been found primarily in larvae. The aldehyde decanal is released primarily by workers and virgin queens (Huang et al. 2009; Winston 1987) and is also present in wax odors. The terpene 3-carene is a major component of wax odors. Methyl benzoate is a floral attractant commonly found inside honeybee colonies (Breed et al. 2004) that is also given off by adult bees. 2-Heptanone is an alarm pheromone component that is released at high rates by disturbed workers (Breed et al. 2004). E-β-ocimene is a volatile primarily given off by egg-laying queens, young larvae, and recently capped brood (Gilley et al. 2006; Maisonnasse et al. 2010). Octanoic acid is a minor component of royal jelly and worker jelly (Nazzi et al. 2009). These six compounds represent a range of volatiles associated with different colony interactions that can be simultaneously sampled with our system.
2 Materials and methods
2.1 Volatile collection system—observation frame
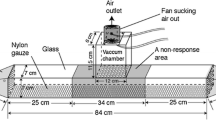
The observation frame is an aluminum and glass plate frame that completely encloses one frame face of active comb (Figure 1a, b). It can be attached directly to an active comb in the field with minimal disturbance to the bees. The rectangular dimensions of the observation frame (20.3 × 43.0-cm inner perimeter) are slightly smaller than the inner perimeter of a standard Langstroth wooden deep frame such that the aluminum frame just contacts the wooden bars of the deep frame. Thus, the observation frame encloses almost all of the brood comb headspace, yet excludes the wooden components of the deep frame. The aluminum frame consists of a 1.91-cm aluminum L-bar welded into a rectangular frame (23.2 ×-51.1 cm outer perimeter). The lower portion of the aluminum frame projects inwards perpendicular to the comb surface. To attach the observation frame to the comb, the inner part of the aluminum frame was sunk vertically through the wax to the underlying foundation (Figure 2a, b). Once embedded to the underlying frame foundation, the dimensions of the aluminum frame maintained uniform bee space (approximately 9 mm) between the glass plate and the brood cells below. For frame drawn deeper than a standard cell depth, the frame can only be partially pressed into the wax at the edges. The upper portion of the aluminum frame forms a flat horizontal flange that extends outwards parallel to the brood comb surface. A plate of a 0.47-cm-thick tempered and beveled safety glass (22.0 × 50.8 cm) was secured against this flange with two 1.27-cm spring clamps (Bessey Tools, Bietigheim-Bissingen, Germany) at opposite ends of the frame to enclose the brood comb headspace. To obtain a better seal between the glass plate and aluminum frame flange, 1.27-cm wide Teflon tape was wrapped around the edge of the glass plate to form a partial gasket.
Fully assembled (a) and exploded front views (b) of the observation frame assembly used to enclose brood comb from a single frame face of a Langstroth deep frame. The observation frame consists of a glass plate (a) overlaying a rectangular aluminum frame (b) pushed into the wax comb of a colony deep frame (c). Access to the airspace of the observation frame is provided by port holes (0.635 cm) (d) drilled through the top. A junction is made across the port by a port connector consisting of interlocking pieces of rigid Teflon tubing (diameter, 0.318–1.588 cm). A frame air line (e) and a frame vacuum line (f) enter the observation frame through separate port connector junctions. A volatile trap consisting of a SuperQ filter (h) and a tube jacket (i) is used to capture odors pulled from the chamber. This trap is placed in-line between a port connector (g) and the frame vacuum line (f). The tube jacket consists of interlocking pieces of rigid Teflon tubing used to secure the SuperQ filter in-line with the vacuum flow (see exploded view).
Fully assembled (a) and exploded cross-section (b) views of the top of the observation frame assembly showing the alignments of the glass plate (a) and aluminum frame (b) relative to the frame foundation (c) and wax cells (d) of the brood comb. The L-bar of the aluminum frame is pushed through the wax to the underlying foundation. e A port connector (0.635 cm) drilled through the aluminum frame provides access across the aluminum frame into the comb headspace. Each port connector is composed of interlocking pieces of rigid Teflon tubing ranging in diameter from 0.318 to 1.588 cm ID. Both airflow and vacuum flow enter the observation frame airspace through these ports. f An extended piece of rigid Teflon tube serves as a port connector extension that directs air and vacuum flow to the center of the comb headspace. Frame air lines are shown as a black line entering the port connector from above.
Access to the frame interior was controlled through ports drilled through the top of the aluminum frame (Figure 2a, b). These ports were drilled along the top of the aluminum frame because bees drop debris into ports placed along the bottom edge. Junctions were made across the port holes with short (3–5 cm) sections of 0.635-cm OD rigid Teflon tubing (Cole-Parmer, Vernon Hills, IL) to form port connectors. Two port connectors served as primary attachment points for an air line and a vacuum line into the frame headspace. Larger diameter pieces of a rigid Teflon tube were used as port connector extensions to direct air and vacuum flow to the center of the comb headspace. One additional port functioned as an access point to provision the enclosed bees with food and water when necessary. Unused ports were partially blocked to prevent bee escape and air intrusion by inserting short sections of smaller diameter (0.318- and 0.476-cm OD) rigid Teflon tubing into the junction. Complete port closure was unnecessary to maintain sample purity because the frame airspace is under positive pressure from the incoming airflow.
2.2 Volatile collection system
Our push–pull airflow system is based on a system originally developed by Heath and Manukian (1994) for the sampling of headspace volatiles from live, undisturbed plants in glass chambers (Figure 3). Regulated air and vacuum flow were passed through the observation frame to allow in-line volatile sampling and provide ventilation to the enclosed bees. House air was filtered (Norgren Excelon CE II GD filters, Norgren USA, Littleton, CO) to remove water and oil contaminants. To provide a microclimate similar to brood nest conditions, airflow was heated and humidified (150 mL bubbler, ARS Glassware, Gainesville, FL, USA) to near colony conditions (32 °C and 60–70 % RH) before entering the observation frame. Both air and vacuum flows were divided between individual frames by a five-flow meter array. An excess of airflow relative to vacuum was delivered to each frame to exclude outside air from the frame headspace. Airflow was set at 681 mL/min and vacuum flow at 515 mL/min by Aalborg P112-02 rotary flow meters (Aalborg, Inc., Orangeburg, NY). To avoid contamination, all air lines from the house air source to the volatile trap were constructed of inert materials such as metal, “clean” plastic (FEP, Teflon), or glass. All vacuum lines downstream of the SuperQ filter holder were made of 0.635-cm Tygon tubing.
Schematic diagram of the push–pull airflow system used to ventilate the observation frame and sample volatiles from the comb headspace. House air and vacuum sources enter the system distal to the air and vacuum reducer valves. The volatile trap (described in Fig. 1b) is placed between the observation frame port and the frame vacuum line. Interconnecting air and vacuum lines are represented by a solid black line. All equipment between the warm water bath and the vacuum flow meter array are housed in an environmental chamber maintained at 32 °C. Only one frame air line (between the airflow meter array and observation frame) and one frame vacuum line (between the volatile trap and the vacuum flow meter array).
Volatiles were collected in a volatile trap containing approximately 50 mg SuperQ 80/100 adsorbent packing material (Sigma, St. Louis, MO) placed in-line between the observation frame port and the frame vacuum line (see insert in Figure 1). SuperQ polymers have been used by many research groups as a relatively unbiased method for the quantification of volatile compounds (D’Alessandro and Turlings 2006). This adsorbent material (now superseded by HayeSep Q 80/100 adsorbent, Hayes Separations, Bandera, TX) readily absorbs a wide array of volatile compounds from airflows actively forced through it. The filter itself consists of the tapered end of a 0.476-cm glass pipette fitted tightly inside 0.635-cm OD rigid Teflon tubing. The adsorbent packing material is trapped between two fine screens and a smaller piece of glass tubing at the bottom of the filter tube. The SuperQ adsorbent filter was placed in-line in a rigid Teflon tube jacket to secure it and protect it from contamination.
The total proportion of volatiles sampled was established at 80 % by a fixed vacuum-to-airflow ratio set by the flow meters. Since the flow ratio was highly dependent on flow meter precision and accuracy, flow meters were frequently calibrated with an external standard flow meter. System flow rates and volatile recovery rates were also checked by releasing and recovering synthetic volatiles in-line by capillary release (D’Alessandro and Turlings 2006). One problem encountered with SuperQ adsorbent is that the packing material affects the flow rate by strongly resisting air passing through it. To establish an accurate flow rate, the flow rates were set with all volatile traps in-line. The volatile traps could then be removed, cleaned with solvent, and reattached before the collection began.
We also considered solid phase microextraction (SPME) as an alternative volatile trapping method for our system. SPME is a thermal desorption method that has been used extensively to characterize honeybee volatiles due to its greater sensitivity and ease of use (Ouyang and Pawliszyn 2008; Sammataro et al. 2009; Schmitt et al. 2007; DeGrandi-Hoffman et al. 2007; Gilley et al. 2006; Nazzi et al. 2004). We exposed SPME fibers for limited periods (20 min to 1 h) in an observation frame through one of the frame port holes. The fiber was partially enclosed in a protective jacket to protect it from coming into contact with the bees. We detected one compound (acetic acid) by SPME hidden by the dichloromethane solvent peak in the SuperQ adsorbent samples. For most compounds, however, collection by SPME was not as sensitive as collection by the SuperQ filter. We therefore decided to leave the development of a SPME-based observation frame sampling system to future studies, in large part because SPME volatile collections are best conducted in environments with limited airflow and because of biases in the absorption of different headspace volatiles by SPME fibers (Agelopoulos and Pickett 1998; Ouyang and Pawliszyn 2008).
2.3 Volatile collection system—environmental chamber
The function of the environmental chamber was to maintain temperatures inside the observation frame at near colony temperatures. Air from the environmental chamber never enters the sealed observation frame or the push–pull airflow system because of the positive pressure airflow system. Rather, the environmental controls of the environmental chamber indirectly affect the air system temperature by regulating the temperature of the observation frame and airflow equipment. In early trials, we observed that air inside the push–pull airflow system was chilled by airflow equipment and air lines kept at ambient room temperatures. The temperature of air flowing through long, narrow constrictions made of conductive material can be readily altered. Critically, chilled air resulted in the condensation of humidified air inside the lines and flow meters. To keep the system heated, the humidifier, airflow meter array, and frame air lines were maintained inside the environmental chamber at 32 °C. Even heating of the environmental chamber was provided by a ceramic heater regulated by a Helix DBS-1000 thermal programmer (Helix Inc., Vista, CA). Heated air was circulated by a small fan throughout the chamber. The entire chamber, except for the front, was insulated by Reflectix bubble foil insulation (Reflectix, Inc., Markleville, IN).
The environmental chamber consisted of a rectangular cube (91.4 × 91.4 × 152.4 cm, H × D × W) constructed of 0.635-cm polyacrylic Plexiglas bolted together by 1.27-cm hex bolts. Access to the front of the chamber was provided by two sliding panels. Holes (0.635–5.080 cm) were drilled through the side walls to provide access for electrical cords, air lines, and vacuum lines from the outside. Lighting was provided by two 60-W incandescent bulbs or 60-W red-coated incandescent bulbs rather than ambient fluorescent lighting in the room. Bees are known to become disoriented by polarized light from fluorescent light bulbs with magnetic ballasts (Pernal and Currie 2001).
2.4 Volatile collections from differently aged larvae
We collected volatiles from single-age larval cohorts to compare volatiles associated with differently aged bee larvae on the comb. Adult worker bees were included as attendants to allow the full range of larvae–worker interactions.
2.5 Insects
Honeybee colonies of European-derived stock were established on Plasticell foundation frame and maintained at the USDA-ARS CMAVE apiary in Gainesville, Florida. These honeybee colonies were not treated with any chemical treatments for mites or diseases from the time of establishment.
2.6 Single-age brood cohorts
Single-age cohorts of honeybee larvae were obtained by caging the queen on a frame face of an empty cell comb. To reduce the complexity of brood frame odors, queens were isolated on newly drawn, empty cell comb completely lacking pollen or nectar stores. Each queen was isolated in a full frame face mesh cage that enclosed her, but allowed passage of worker bees through the mesh. Each oviposition frame was placed in the brood center of the colony to maximize attendance by well-fed frame bees. Queens were allowed to oviposit for 16–20 h overnight and were then removed to a distant part of the colony to prevent further oviposition on the frame. New oviposition frames were then reared in the brood center of the colony flanked by frames containing open brood and abundant pollen stores. Special precautions were required to obtain even and predictable brood development patterns. To ensure similar microclimatic and brood care conditions across the whole oviposition frame, single-age brood cohorts were established in large double deep colonies (30,000 to 60,000 adult bees) during periods where nighttime temperatures remained above 20 °C.
2.7 Preparation of observation frames for volatile collections
For each larval age group, we sampled three replicates (each consisting of a single-age cohort established on a single frame face) from separate colonies. Each brood cohort construct was sampled only once. The number of larvae present in the brood patches ranged from 162 to 242 and the number of attending adult bees ranged from 89 to 116. Observation frames were cleaned between uses first by scraping off any wax, honey, or propolis with a razor under hot water. Frames were then dried off before being wiped down with acetone-soaked paper towels and baked at 90 °C in a drying oven.
Only fully drawn frames with Plasticell polystyrene foundation were used in our volatile collections. Wax foundation did not provide enough support and softer plastic foundations gave off complex mixtures of contaminant volatiles. Frame faces with brood in cells on the extreme periphery (one to four cells from the wooden bars) were excluded to avoid sampling injury volatiles. We selected colony frames that were fully drawn to the edge without large gaps between the wooden frame and its underlying foundation. Small gaps were plugged with clean wax taken from the same colony.
Observation frames were attached to the comb directly in the field. All frame attachments were conducted on cool surfaces in the shade to prevent overheating the bees. The colony frame was placed horizontally on a tilted surface to avoid crushing the bees underneath. We took precautions to avoid crushing adult bees between the aluminum frame and the colony frame bars. Once proper alignment and clearance was achieved, the observation frame was pushed through the wax comb until it contacted the underlying frame foundation. The observation frame was further secured to the brood frame by large rubber bands. Excess bees were swept off the outside of the frame with a bee brush. The observation frame was then quickly brought into the environmental chamber and mounted on a Plexiglas frame holder. A volatile trap was attached in-line between a frame port and the vacuum line to begin sampling. Volatiles were collected for 3 h. Four hundred nanograms nonyl acetate in 5 μL dichloromethane was added as an internal standard to the adsorbent packing of the filter just before elution. Adsorbed volatiles were then eluted from the SuperQ with 200 μL dichloromethane.
2.8 GC-MS analysis
Volatile analysis was performed by positive ion electron impact gas chromatography–mass spectrometry (EI GC-MS) on an HP 6890 gas chromatograph coupled to an HP 5973 mass spectrometer detector. One microliter of each sample solution was injected at 240 °C onto an Agilent HP-5MS column (30-m × 0.250-mm ID × 0.250-μm film; Agilent Technologies, Santa Clara, CA) and separated by oven temperatures programmed from 35 °C (1.0 min hold) to 230 °C at 10 °C/min and held for 5 min. Injections were conducted in splitless mode to maximum compound sensitivity. Helium was employed as a carrier gas at a flow rate of 1.2 mL/min. Sample compounds were identified by (a) comparison of the mass spectra with the mass spectra libraries (NIST and Department of Chemical Ecology, Göteburg University, Sweden) and (b) comparison of the retention times and the mass spectra with authentic standards (Sigma). Confirmation of compound identities was provided by injecting the samples and authentic standards on an Agilent DB-35MS column (30-m × 0.250-mm ID × 0.25-μm film; Agilent Technologies) under similar chromatography conditions.
Volatile emission rates were calculated by comparing the peak area of each compound with the peak area of the nonyl acetate internal standard. The emission rates were adjusted to reflect differences in the air and vacuum flow rates. For all samples, only 80 % of the air pushed into the frame headspace was pulled through the filter by the vacuum. The emission rates were placed on a per brood individual standing based on counts of brood during the collection or counts from photos of brood frames. For each compound, the volatile emission rates were compared across ages of larvae by non-parametric Kruskal–Wallis tests. Statistical analyses were performed using the NPAR1WAY procedures of SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
2.9 Background odors associated with comb
One of the major problems with sampling volatiles off active comb is the abundance of background odors. Nectar, pollen, and plant resins that bees collect all are exceptionally rich in volatile plant secondary compounds. Floral resources release a myriad of odor compounds to attract pollinators, while resins contain rich mixtures of antimicrobial and antiherbivore volatile compounds such as terpenes (Dobson 1994; Pernal and Currie 2002; Woisky and Salatino 1998). In addition, the odor profiles of plant resources change as these materials are processed into honey, bee bread, and propolis. Nectar and pollen are extensively colonized by microbial organisms that give off volatiles as these food materials are converted into honey and bee bread (Anderson et al. 2011). Furthermore, nectar, pollen, and resin sources vary as the abundance of particular floral and resin sources changes through the season. Finally, wax comb contains many odor compounds both native and absorbed (Winston 1987). As a matrix of hydrocarbons, long-chain esters, acids, and alcohols, beeswax readily absorbs, stores, and disperses lipophilic volatile compounds from stored food materials and brood. These odors may persist in the comb matrix for some time after the material is removed from the colony.
For these reasons, it is important to sample representative comb from experimental colonies to identify background odors associated with comb from a particular time and location. We sample background odors from pollen, nectar, and wax to identify major background odors from these materials. To limit the complexity of background odors from our brood samples, we only use empty cell comb (wax only) to set up single-age brood cohorts rather than comb with bee bread, nectar, honey, or a history of brood. By the time volatiles are collected, nectar has generally been added to the comb periphery so that enclosed bees still have an energy source.
3 Results
Enclosed brood comb containing active larvae and attending workers yielded a broad array of over 40 colony-related volatiles (Figure 4a, b and Table I). Among the volatiles detected and identified were carboxylic acids (isobutyric acid, butyric acid, pentanoic acid, hexanoic acid, octanoic acid); aromatic acids (benzoic acid); phenylpropanoids (methyl benzoate, ethyl benzoate); terpenoids (alpha-pinene, 3-carene, E-β-ocimene, citral, nerol, geraniol); aldehydes (hexanal, heptanal, octanal, nonanal, decanal); ketones (2-pentanone, 2-heptanone, 6-methyl-5-hepten-2-one); alcohols (3-methyl-1-butanol, 2-heptanol); esters (isobutyl acetate, isopentyl acetate); and alkanes (nonane and decane). The volatiles identified here were detected in all brood comb containing larvae and adult workers. The volatile emissions of these colony volatiles therefore varied quantitatively rather than absolutely in combs containing differently aged larvae.
Representative gas chromatograms (GC-MS) of volatiles sampled from empty cell comb (a) and brood comb (b) containing 162–242 larvae (5–6 days old) and 89–116 adults during a 3-h collection. Bees in the brood comb were provisioned with sucrose sugar syrup and water, both of which are relatively odorless for the six compounds examined here. For the empty cell comb odors, we sampled comb that had previously been used to rear brood to present the full range of background odors typically present in brood comb. Volatile compounds identified here include (1) 2-heptanone (6.372 min RT), (2) 3-carene (8.560 min RT), (3) E-β-ocimene (9.102 min RT), (4) methyl benzoate (9.640 min RT), (5) octanoic acid (10.848 min RT), (6) decanal (11.372 min RT), and (7) nonyl acetate (12.890 min RT, an internal standard added to the sample). NP indicates that the compound was not detected or in trace amounts. The chromatogram is displayed in total ion mode from m/z 60–550 and was run in splitless mode on EI GC-MS.
Of the six volatiles we examined, only 3-carene and decanal were present in the empty cell wax comb (blank frame; Figure 4a). 2-Heptanone and methyl benzoate were emitted in trace amounts by adult bees isolated on empty cell wax comb. The volatile emission patterns of the six individual volatiles varied among comb containing larvae of different ages (Figure 4b). The emissions of the four volatiles associated with adult workers or colony materials were generally highest from comb containing older larvae (Figure 5). Volatile emissions of the wax component 3-carene, the floral fragrance methyl benzoate, and the adult bee aldehyde decanal did not significantly vary across comb with differently aged larvae (Kruskal–Wallis test: 3-carene, n = 3, df = 5, H = 2.637, P = 0.756; methyl benzoate, n = 3, df = 5, H = 5.561, P = 0.351; decanal, n = 3, df = 5, H = 4.322, P = 0.504; Figure 5). The production of the alarm pheromone component 2-heptanone marginally did not vary significantly among differently aged constructs (Kruskal–Wallis test: n = 3, df = 5, H = 10.742, P = 0.057; Figure 5). 2-Heptanone was emitted at the lowest amounts among comb containing the youngest (3–4 days) larvae and steadily increased in comb with older larvae through capped brood (8–9 days; Figure 5).
Emission rates of selected headspace volatiles collected from observation frames containing bee larvae in different stages of development. Each observation frame enclosed between 162 and 242 larvae (5–6 days old) and from 89 to 116 attending adult workers. Compounds with volatile emissions that varied significantly in comb containing differently aged larvae are indicated by an asterisk (Kruskal–Wallis test, n = 3, P < 0.05). The mean compound emission rates are reported here with standard error indicated by error bars.
Volatile emissions of the larval pheromone E-β-ocimene followed a bimodal pattern (Kruskal–Wallis test: n = 3, df = 5, H = 12.790, P = 0.025; Figure 5). E-β-ocimene emissions were low in comb containing newly hatched larvae (3–4 days). Emissions were higher among slightly older larvae (4–5, 5–6, and 6–7 days), then declined sharply in comb containing capping larvae (7–8 days). E-β-ocimene production rose sharply again in comb containing 8- to 9-day-old capped brood.
Octanoic acid emissions varied significantly among combs with differently aged larvae (Kruskal–Wallis test: n = 3, df = 5, H = 14.591, P = 0.012; Figure 5). The production of the larval food component octanoic acid was highest in comb containing 4- to 5-day-old larvae and declined with larval age through comb containing freshly capped brood (8–9 days). Octanoic acid emissions were also low in comb containing newly hatched larvae (3–4 days).
4 Discussion
It is critical to recognize that volatiles collected from brood comb headspace represent volatiles associated with, but not necessarily originating from, the larvae themselves. These volatiles, like many honeybee compounds, may have unknown roles and odor sources within the colony. While admittedly more complicated than sampling from isolated individuals, in situ sampling captures volatiles present in the normal environment of the individual. Such an approach will provide insight into the interactions between colony members as they occur on the brood comb.
Notably, many of the volatiles emitted from the brood comb headspace are not produced by the larvae themselves. The volatiles 2-heptanone, 3-carene, methyl benzoate, and decanal are known to either be emitted by adult worker bees or present in materials manipulated by worker bees (Torto et al. 2007). Interestingly, the emissions of these volatiles all showed a trend toward higher release rates on brood frames containing older larvae. As odors from colony materials, the monoterpene 3-carene and the phenylpropanoid methyl benzoate are both commonly dispersed through the colony. Our frame constructs lacked the pollen and nectar stores that often contain high levels of methyl benzoate, yet the compound was present in all our collections. The alarm component 2-heptanone was also emitted at low levels characteristic of undisturbed bees. Comb with older larvae emitted higher amounts of decanal than comb with younger larvae. Decanal is produced by both queen and worker honeybees. Although higher emissions have been observed from virgin queens, the role of this volatile in adult and larval bees remains unknown (Huang et al. 2009; Winston 1987).
In contrast to adult bee volatiles, larval food volatile emissions would be expected to reflect changes in food production by adult workers and consumption among larvae. Octanoic acid is a known component of bee larval food that occurs at a much higher concentration in royal jelly (~40 times) than drone food or older worker food (Nazzi et al. 2009). The emission rates were higher from frames containing younger larvae (4–5 to 5–6 days after oviposition) than older larvae despite the fact that younger larvae are fed less food than their older counterparts (Schmick and Crailsheim 2002; Haydak 1970). The emission patterns observed here may be attributed to differences in the composition of larval food fed to younger and older larvae. Larval food of younger larvae probably emits higher levels of octanoic acid because it contains a higher percentage of worker jelly than the brood food fed to older larvae (Huang and Otis 1991). Young larvae are mass provisioned with worker jelly that they consume slowly, increasing the time the food is exposed to the atmosphere (Haydak 1970). In contrast, nurse bees frequently feed small amounts of gland secretions to older larvae which rapidly consume the provisions (Jung-Hoffmann 1966; Haydak 1970). The effects of larval age on the octanoic acid emissions of food-producing adults are not clear and may represent a major emission source for this volatile. Notably, these increases in octanoic acid emissions would probably not have been observed in volatile collections made from isolated larvae.
Our collections of the terpene E-β-ocimene closely follow the emission patterns reported by Maisonnasse et al. (2009, 2010). These authors quantified E-β-ocimene emissions by collecting volatiles with SPME from isolated larvae in glass jars. This terpene appears to be intimately involved in the regulation of the nutritional balance in the colony. E-β-ocimene acts as a primer pheromone on young adult bees by accelerating the maturation of nurse bees into foragers and suppressing ovary development. Younger bees benefit from an increase in foragers by increased food availability. Curiously, both research groups observed high emission rates of E-β-ocimene among recently capped larvae. Compared to the uncapped fifth-instar larvae that immediately preceded them, recently capped larvae have a greatly reduced need for brood care from nurse bees. Acceleration of adult forager maturation may also benefit recently capped individuals by increasing the amount of food available later when the new adult bee emerges. Likewise, recently capped larvae no longer benefit from the feeding patterns induced by brood pheromone, a larval pheromone that counters the pheromonal action of E-β-ocimene (Maisonnasse et al. 2010).
Brood comb volatiles can also be used as markers to track disturbance in frame preparations. The honeybees in our observation frame constructs generally emitted relatively negligible amounts of 2-heptanone and other alarm pheromone components, indicative of low disturbance levels (Breed et al. 2004). In contrast, we have observed that visibly disturbed bees enclosed in a cylindrical glass container lacking a comb structure release much higher levels of alarm pheromone components. We believe that 2-heptanone can be monitored in observation frame preparations as an index of adult worker disturbance. The volatile emissions of 2-heptanone in our collections were relatively low, except for one frame containing 7- to 8-day-old capping larvae that emitted almost ten times the amount of other similar frames. A closer examination of this frame’s history noted that this colony had been entered multiple times in 1 week by our beekeepers and probably contained highly disturbed worker bees. Honeybees emit higher background levels of alarm pheromones after disturbance (Torto et al. 2007).
Our results confirm that chemical interactions in honeybee colonies occur in a chemically complex environment. In our sampling of background odors, we detected two volatiles (decanal and 3-carene) from wax and two volatiles (2-heptanone and methyl benzoate) from adult bees. While some would regard the presence of these volatiles in background odors as a confounding factor, we believe that these checks provide a realistic context for chemical signaling. Semiochemicals in honeybee colonies do not operate in isolation, but function against an ever-changing backdrop of related odors. Honeybee responses to semiochemical cues are thought to be triggered by threshold responses to stimuli (Pankiw 2003; Nowak et al. 2010). Such mechanisms make sense when many semiochemicals are present in the colony in at least trace amounts from previous interactions and constitutive releases (alarm pheromone in Torto et al. 2007). Through careful selection and comparison of background samples, our method can be used to piece together the source of background odors.
We also emphasize that while our method detects volatile compounds, it does not confirm a mode of action as a volatile. Compounds detected on a mass scale as dispersed frame odors may act at the level of a localized interaction in a more concentrated form. For example, while several components of the queen retinue pheromone complex are clearly volatile enough to be separated by GC-MS, QRP appears to operate primarily by contact in the colony (Kaminski et al. 1990). Further experimentation is required to determine the chemical source of a volatile in the colony as well as the primary mode of activity in bees, if any. However, this method provides a rapid way to detect differences in volatile emissions between bees in a natural setting.
These methods provide a thorough sampling of headspace volatiles from intact brood comb containing relatively undisturbed bees. Fully 80 % of the volatile emissions released from brood frame bees and structures are captured by our push–pull airflow system, as demonstrated by the in-line release of a synthetic internal standard (data not shown). The large number of bees present on the brood comb ensures that the sample represents a pooled sample of the volatiles emitted by many bees. Estimations of emission rates can be further refined by adjusting for the number of bees (brood or adult) present on the comb. Both brood numbers and adult numbers can be estimated either by direct observation or counted from still photographs. The large number of potential odor sources enclosed on a frame face usually allows for the identification of minor components or background emissions that might remain undetected in smaller sample sizes. Our approach allows for the calculation of biologically relevant rates of volatile emissions, provided that the source emission is continuous or frequent enough. Volatile collection times generally range from 30 min to 24 h, with 3 h being the most common sampling period. Our method works best when sampling moderately long or progressive events, such as larval development.
A useful feature of our collection system is the ability to rapidly enclose bees with a minimal disturbance of normal colony functions. As a test, we maintained an egg-laying queen and brood-rearing workers on a comb face over a 16-day period. During this time, normal colony activities such as oviposition continued and eggs were reared through to capped brood. In practice, we try to isolate bees for no more than several hours to limit the unknown effects of isolation from the colony and outside surroundings. In particular, worker bees isolated without a queen are gradually deprived of queen mandibular pheromones (Naumann et al. 1991). Enclosed bees also cannot engage in outside activities—we frequently observed isolated forager and undertaker bees moving rapidly around the comb searching for an exit.
The applications for the observation frame volatile collection system are not limited to the characterization of brood volatiles alone. The system described here can be used to investigate any chemical exchange that occurs on a comb face at a mass scale. Since its conception, the observation frame volatile collection system has been used to examine the chemical underpinnings of disease etiology, pheromones, nutrition, food processing, and host–parasite interactions in the colony environment. These investigations with the observation frame volatile collection system will continue to yield biologically relevant information about chemical interactions in the hive environment.
References
Agelopoulos, N.G., Pickett, J.A. (1998) Headspace analysis in chemical ecology: effects of different sampling methods on ratios of volatile compounds present in headspace samples. J. Chem. Ecol. 24, 1161–1172
Alaux, C., Maisonanasse, A., Le Conte, Y. (2010) Pheromones in a superorganism: from gene to social regulation. Vitam. Horm. 83, 401–423
Anderson, K.E., Sheehan, T.H., Eckholm, B.J., Mott, B.M., DeGrandi-Hoffman, G. (2011) An emerging paradigm of colony health: microbial balance of the honey bee and hive (Apis mellifera). Insectes Soc. 58, 431–444
Barbier, J., Lederer, E. (1960) Structure chimique de la substance royale de la reine d’abeille (Apis mellifera L.). C. R. Acad. Sci., Ser. 3 Sci. 251, 1131–1135
Blum, M.S., Fales, H.M. (1988) Eclectic chemisociality of the honeybee: a wealth of behaviors, pheromones, and exocrine glands. J. Chem. Ecol. 14, 2099–2107
Boch, R., Shearer, A., Petrasovits, A. (1970) Efficacies of two alarm substances of the honey bee. J. Insect Physiol. 16, 17–24
Breed, M.D., Guzman-Novoa, E., Hunt, G.J. (2004) Defensive behavior of honey bees: organization, genetics, and comparisons with other bees. Annu. Rev. Entomol. 49, 271–298
Collins, A.M., Blum, M.S. (1983) Alarm responses caused by newly identified compounds derived from the honeybee sting. J. Chem. Ecol. 9, 57–65
D’Alessandro, M., Turlings, T.C.J. (2006) Advances and challenges in the identification of volatiles that mediate interactions among plants and arthropods. Analyst 131, 24–32
DeGrandi-Hoffman, G., Gilley, D., Hooper, J. (2007) The influence of season and volatile compounds on the acceptance of introduced European honey bee (Apis mellifera) queens into European and Africanized colonies. Apidologie 38, 230–237
Dobson, H.E.M. (1994) Floral volatiles in insect biology. In: Bernays, E.A. (ed.) Insect–Plant Interactions, vol. 5, pp. 47–81. CRC, Boca Raton
Dussaubat, C., Maisonnase, A., Alaux, C., Tchamitchan, S., Brunet, J.L., Plettner, E., Belzuncces, L.P., Le Conte, Y. (2010) Nosema spp. infection alters pheromone production in honey bees (Apis mellifera). J. Chem. Ecol. 36, 522–525
Free, J.B., Winder, M.E. (1983) Brood recognition by honey bee Apis mellifera workers. Anim. Behav. 31, 539–545
Garrido, C., Rosenkranz, P. (2004) Volatiles of the honey bee larva initiate oogenesis in the parasitic mite Varroa destructor. Chemoecology 14, 193–197
Gilley, D.C., DeGrandi-Hoffman, G., Hooper, J.E. (2006) Volatile compounds emitted by live European honey bee (Apis mellifera L.) queens. J. Insect. Physiol. 52, 520–527
Gramacho, K.P., Spivak, M. (2003) Differences in olfactory sensitivity and behavioral responses among honey bees bred for hygienic behavior. Behav. Ecol. Sociobiol. 54, 472–479
Haydak, M.H. (1970) Honey bee nutrition. Annu. Rev. Entomol. 15, 143–156
Heath, R.R., Manukian, A. (1994) An automated system for use in collecting volatile chemicals released from plants. J. Chem. Ecol. 20, 593–608
Huang, Z.Y., Otis, G.W. (1991) Inspection and feeding of larvae by worker honey bees (Hymenoptera: Apidae): effect of starvation and food quality. J. Insect Behav. 4, 305–317
Huang, M.H., DeGrandi-Hoffman, G., LeBlanc, B. (2009) Comparisons of the queen volatile compounds of instrumentally inseminated versus naturally mated honey bee (Apis mellifera) queens. Apidologie 40, 464–471
Hunt, G.J. (2007) Flight and fight: a comparative view of the neurophysiology and genetics of honey bee defensive behavior. J. Insect Physiol. 53, 399–410
Jung-Hoffmann, I. (1966) Die Determination von Königin und Arbeiterin der Honingbiene. Z Bienenforsch 8, 296–322
Kaminski, L.A., Slessor, K.N., Winston, M.L., Hay, N., Borden, J.H. (1990) Honey bee responses to queen mandibular pheromone in laboratory bioassays. J. Chem. Ecol. 16, 841–850
Keeling, C.I., Slessor, K.N., Higo, H.A., Winston, M.L. (2003) New components of the honey bee (Apis mellifera L.) queen retinue pheromone. PNAS 100, 4486–4491
Le Conte, Y., Arnold, G., Trouiller, J., Masson, C., Chappe, B., Ourisson, G. (1989) Attraction of the parasitic mite Varroa to the drone larvae of honey bees by simple aliphatic esters. Science 245, 638–639
Le Conte, Y., Arnold, G., Trouiller, J., Masson, C. (1990) Identification of a brood pheromone in honeybees. Naturwissenschaften 81, 462–465
Maisonnasse, A., Lenoir, J.C., Costagliola, G., Beslay, D., Choteau, F., Crauser, D., Becard, J.-M., Plettner, E., LeConte, Y. (2009) A scientific note on E-β ocimene, a new volatile primer pheromone that inhibits worker ovary development in honey bees. Apidologie 40, 562–564
Maisonnasse, A., Lenior, J.-C., Beslay, D., Crauser, D., LeConte, Y. (2010) E-β-ocimene, a volatile brood pheromone involved in social regulation in the honey bee colony (Apis mellifera). PLoS One 5, 1–7
Naumann, K., Winston, M.L., Slessor, K.N., Prestwich, G.D., Webster, F.X. (1991) Production and transmission of honey bee queen (Apis mellifera L.) mandibular gland pheromone. Behav. Ecol. Sociobiol. 29, 321–332
Nazzi, F., Milani, N., Della Vedova, G., Nimis, M. (2004) Semiochemicals from larval food affect the locomotory behavior of the Varroa mite. Apidologie 32, 149–155
Nazzi, F., Bortolomeazzi, R., Vedova, G.D., Del Piccolo, F., Annoscia, D., Milani, N. (2009) Octanoic acid confers to royal jelly Varroa-repellent properties. Naturwissenschaften 96, 309–314
Nowak, M.A., Tarnita, C.E., Wilson, E.O. (2010) The evolution of eusociality. Nature 466, 1057–1062
Ouyang, G., Pawliszyn, J. (2008) A critical review in calibration methods for solid-phase microextraction. Anal. Chim. Acta 627, 184–197
Palacio, M.A., Rodriguez, E., Goncalves, L., Bedascarrasbure, E., Spivak, M. (2010) Hygienic behaviors of honey bees in response to brood experimentally pin-killed or infected with Ascosphaera apis. Apidologie 41, 602–612
Pankiw, T. (2003) Directional change in a suite of foraging behaviors in tropical and temperate evolved honeybees (Apis mellifera L.). Behav. Ecol. Sociol. 54, 458–464
Pankiw, T. (2004) Cued in: honey bee pheromones as information flow and collective decision-making. Apidologie 35, 217–226
Pernal, S.F., Currie, R.W. (2001) Improved flight and rearing room design for honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 94, 793–805
Pernal, S.F., Currie, W. (2002) Discrimination and preferences for pollen-based cues by foraging honeybees, Apis mellifera L. Anim. Behav. 63, 369–390
Pickett, J.A., Williams, I.H., Martin, A.P. (1982) (Z)-11-eicosen-1-ol, an important new pheromonal component from the sting of the honey bee, Apis mellifera L. (Hymenoptera, Apidae). J. Chem. Ecol. 8, 163–175
Sammataro, D., Finley, J., LeBlanc, B., Wardell, G., Ahumada-Segura, F., Carroll, M.J. (2009) Feeding essential oils and 2-heptanone in sugar syrup and liquid protein diets to honey bees (Apis mellifera L.) as potential Varroa mite (Varroa destructor) controls. J. Apic. Res. 48, 256–262
Schmick, T., Crailsheim, K. (2002) How honeybees (Apis mellifera L.) change their broodcare behaviour in reponse to non-foraging conditions and poor pollen conditions. Behav. Ecol. Sociobiol. 51, 415–425
Schmitt, T., Herzner, G., Weckerle, B., Schreier, P., Strohm, E. (2007) Volatiles of foraging honeybees Apis mellifera (Hymenoptera: Apidae) and their potential roles as semiochemicals. Apidologie 38, 164–170
Shearer, D.A., Boch, R. (1962) Identification of geraniol as the active component in the Nassanoff pheromone of the honey bee. Nature 194, 704–706
Shearer, D.A., Boch, R. (1965) 2-Heptanone in the mandibular gland secretion of the honey-bee. Nature 206, 530
Slessor, K.N., Kaminski, L.A., King, G.G.S., Borden, J.H., Winston, M.L. (1988) Semiochemical basis of the retinue response to queen honey bees. Nature 24, 354–356
Slessor, K.N., Winston, M.L., LeConte, Y. (2005a) Pheromone communication in the honey bee (Apis mellifera L.). J. Chem. Ecol. 31, 2731–2745
Slessor, K.N., Winston, M.L., Le Conte, Y. (2005b) Pheromone communication in the honeybee (Apis mellifera L.). J. Chem. Ecol. 31, 2731–2745
Stout, J.C., Goulson, D. (2001) The use of conspecific and interspecific scent marks by foraging bumblebees and honeybees. Anim. Behav. 62, 183–189
Swanson, J.A.I., Torto, B., Kells, S.A., Mesce, K.A., Tumlinson, J.H., Spivak, M. (2009) Odorants that induce hygienic behavior in honeybees: identification of volatile compounds in chalkbrood-infected honeybee larvae. J. Chem. Ecol. 35, 1108–1116
Thom, C., Gilley, D.C., Hooper, J., Esch, H.E. (2007) The scent of the waggle dance. PLoS Biol. 5, 1862–1867
Torto, B., Boucias, D.G., Arbogast, R.T., Tumlinson, J.H., Teal, P.E.A. (2007) Multitrophic interaction facilitates parasite–host relationship between an invasive beetle and the honey bee. PNAS 104, 8374–8378
Vallet, A., Cassier, P., Lensky, Y. (1991) Ontogeny of the fine structure of the mandibular glands of the honeybee (Apis mellifera L.) workers and the pheromonal activity of 2-heptanone. J Insect Physiol. 37, 789–804
Winston, M.L. (1987) The Biology of the Honey Bee. The Harvard Press, Cambridge
Woisky, R.G., Salatino, A. (1998) Analysis of propolis: some parameters and procedures for chemical quality control. J. Apic. Res. 37, 99–105
Acknowledgments
The authors would like to thank Steve Willms, Eric Schmelz, Hans Alborn, and Peter Teal for critical insight and technical contributions to the design of this volatile collection system.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript editor: Monique Gauthier
Collecte des substances volatiles émises par les larves et adultes d’abeilles et présentes sur les cadres de couvain. Apis mellifera /substances volatiles / composé sémiochimique / phéromone/larve
Die Sammlung flüchtiger Duftstoffe von Larven und auf Brutwaben eingeschlossenen adulten Honigbienen. Apis mellifera /volatile Stoffe / Kommunikationsduftstoffe / Pheromone / Larven
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Carroll, M.J., Duehl, A.J. Collection of volatiles from honeybee larvae and adults enclosed on brood frames. Apidologie 43, 715–730 (2012). https://doi.org/10.1007/s13592-012-0153-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-012-0153-x