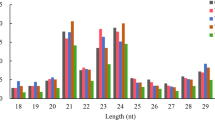
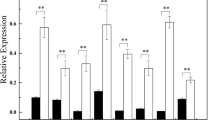
Abstract
Grafting can improve the growth and abiotic stress tolerance of cucumber. MicroRNAs (miRNAs) play important roles in regulating plant growth and development and in various stress responses. The aim of this study was to investigate the molecular mechanisms of miRNA-mediated responses to nitrogen- (N) and phosphorus- (P) deficiency in grafted cucumber (Cucumis sativus). Cucumber scions were grafted onto pumpkin (Cucurbita moschata) or C. sativus rootstocks, to yield hetero- and auto-grafted plants, respectively, and the expression of 19 miRNAs and their predicted target mRNAs under N- or P-deficiency were detected by quantitative real-time PCR. Compared with auto-grafted seedlings, hetero-grafted seedlings showed higher expression levels of most miRNAs. In the leaves of hetero-grafted cucumber seedlings, the expression of most miRNAs was increased by 24 and 48 hours of N-deficiency, but decreased by 24 and 48 hours of P-deficiency. In the roots of hetero-grafted seedlings, the expression level of most miRNAs was increased by 24 hours of N-deficiency and 48 hours of P-deficiency. In the leaves of hetero-grafted cucumber seedlings, the expression levels of most of the miRNA-target genes were increased by 24 and 48 hours of N-deficiency, but decreased by 24 and 48 hours of P-deficiency. In the roots, the expression levels of most miRNA-target genes were higher in hetero-grafted seedlings than in auto-grafted seedlings. Under N- or P-deficiency, most target genes showed markedly increased expression levels in the roots of hetero-grafted cucumber seedlings. Our results will be useful for dissecting the miRNA-mediated enhanced tolerance to N- and P-deficiency in grafted cucumber seedlings.
Similar content being viewed by others
Literature Cited
Achard, P., A. Herr, D.C. Baulcombe, and N.P. Harberd. 2004. Modulation of floral development by a gibberellin-regulated microRNA. Development 131;3357–3365.
Allen, E., Xie, Z., Gustafson, A. M., & Carrington, J. C. 2005. microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121;207–221.
Aukerman, M.J. and H. Sakai. 2003. Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15;2730–2741.
Axtell, M.J. and D.P. Bartel. 2005. Antiquity of microRNAs and their targets in land plants. Plant Cell 17;1658–1673.
Bari, R., B.D. Pant, M. Stitt, and W.-R. Scheible. 2006. PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol. 141;988–999.
Bartel, D.P. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116;281–297.
Brodersen, P., L. Sakvarelidze-Achard, M. Bruun-Rasmussen, P. Dunoyer, Y.Y. Yamamoto, L. Sieburth, and O. Voinnet. 2008. Widespread translational inhibition by plant miRNAs and siRNAs. Science 320;1185–1190.
Chiou, T. and S.I. Lin. 2011. Signaling network in sensing phosphate availability in plants. Annu. Rev. Plant Biol. 62;185–206.
Chiou, T.J., K. Aung, S.-I. Lin, C.C. Wu, S.F. Chiang, and C.-l. Su. 2006. Regulation of phosphate homeostasis by microRNA in Arabidopsis. Plant Cell 18;412–421.
Colla, G., C.M.C. Suárez, M. Cardarelli, and Y. Rouphael. 2010. Improving nitrogen use efficiency in melon by grafting. HortScience 45;559–565.
Dai X.B. and Zhao P.X.C. 2011. psRNATarget: a plant small RNA target analysis server. Nucleic Acids Res. 39;155–159.
Devaiah, B.N., R. Madhuvanthi, A.S. Karthikeyan, and K.G. Raghothama. 2009. Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Mol. Plant 2;43–58.
Ding, D., L. Zhang, H. Wang, Z. Liu, Z. Zhang, and Y. Zheng. 2009. Differential expression of miRNAs in response to salt stress in maize roots. Ann. Bot. 103;29–38.
Edelstein, M., R. Cohen, Y. Burger, S. Shriber, S. Pivonia, and D. Shtienberg. 1999. Integrated management of sudden wilt in melons, caused by Monosporascus cannonballus, using grafting and reduced rates of methyl bromide. Plant Dis. 83;1142–1145.
Estañ, M.T., M.M. Martinez-Rodriguez, F. Perez-Alfocea, T.J. Flowers, and M.C. Bolarin. 2005. Grafting raises the salt tolerance of tomato through limiting the transport of sodium and chloride to the shoot. J. Exp. Bot. 56;703–712.
Floyd, S. K., and Bowman, J. L. 2004. Gene regulation: ancient microRNA target sequences in plants. Nature 428;485–486.
Fujii, H., T.J. Chiou, S.I. Lin, K. Aung, and J. Zhu. 2005. A miRNA involved in phosphate-starvation response in Arabidopsis. Curr. Biol. 15;2038–2043.
García Sánchez, F., J. Syvertsen, V. Gimeno, P. Botía, and J.G. Perez Perez. 2007. Responses to flooding and drought stress by two citrus rootstock seedlings with different water-use efficiency. Physiol. Plant. 130;532–542.
Gifford, M.L., A. Dean, R.A. Gutierrez, G.M. Coruzzi, and K.D. Birnbaum. 2008. Cell-specific nitrogen responses mediate developmental plasticity. Proc. Natl. Acad. Sci. USA 105;803–808.
Jagadeeswaran, G., A. Saini, and R. Sunkar. 2009. Biotic and abiotic stress down-regulate miR398 expression in Arabidopsis. Planta 229;1009–1014.
Jones-Rhoades, M.W. and D.P. Bartel. 2004. Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol. Cell 14;787–799.
Kant, S., Y. Bi, and S.J. Rothstein. 2011. Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 62;1499–1509.
Kawashima, C.G., N. Yoshimoto, A. Maruyama Nakashita, Y.N. Tsuchiya, K. Saito, H. Takahashi, and T. Dalmay. 2009. Sulphur starvation induces the expression of microRNA395 and one of its target genes but in different cell types. Plant J. 57;313–321.
Khraiwesh, B., J. Zhu, and J. Zhu. 2012. Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. Biochim. Biophys. Acta, Gene Regul. Mech. 1819;137–148.
Kuo, H. and T.J. Chiou. 2011. The role of microRNAs in phosphorus deficiency signaling. Plant Physiol. 156;1016–1024.
Kurihara, Y. and Y. Watanabe. 2004. Arabidopsis microRNA biogenesis through Dicer-like 1 protein functions. Proc. Natl. Acad. Sci. USA 101;12753–12758.
Lexa, M. and J.M. Cheeseman. 1997. Growth and nitrogen relations in reciprocal grafts of wild-type and nitrate reductase-deficient mutants of pea (Pisum sativum L. var. Juneau). J. Exp. Bot. 48;1241–1250.
Li, C.H., Y.S. Li, L.Q. Bai, T.Y. Zhang, C.X. He, Y. Yan, and X.C. Yu. 2014. Grafting-responsive miRNAs in cucumber and pumpkin seedlings identified by high-throughput sequencing at whole genome level. Physiol. Plant. 151;406–422.
Li, W., Y. Oono, J. Zhu, X. He, J. Wu, K. Iida, X. Lu, X. Cui, H. Jin, and J. Zhu. 2008. The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell 20;2238–2251.
Liang, G., H. He, and D. Yu. 2012. Identification of nitrogen starvation-responsive microRNAs in Arabidopsis thaliana. PLoS ONE 7;e48951.
Liu, K.H., C.Y. Huang, and Y.F. Tsay. 1999. CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell 11;865–874.
Liu, N., J. Yang, S. Guo, Y. Xu, and M. Zhang. 2013. Genome-wide identification and comparative analysis of conserved and novel microRNAs in grafted watermelon by high-throughput sequencing. PLoS ONE 8;e57359.
Meng, Y., X. Ma, D. Chen, P. Wu, and M. Chen. 2010. MicroRNAmediated signaling involved in plant root development. Biochem. Biophys. Res. Commun. 393;345–349.
Miyake, K., T. Ito, M. Senda, R. Ishikawa, T. Harada, M. Niizeki, and S. Akada. 2003. Isolation of a subfamily of genes for R2R3-MYB transcription factors showing up-regulated expression under nitrogen nutrient-limited conditions. Plant Mol. Biol. 53;237–245.
Moore, R. 1982. Graft formation in Kalanchoe blossfeldiana. J. Exp. Bot. 33;533–540.
Pant, B.D., M. Musialak-Lange, P. Nuc, P. May, A. Buhtz, J. Kehr, D. Walther, and W.-R. Scheible. 2009. Identification of nutrientresponsive Arabidopsis and rapeseed microRNAs by comprehensive real-time polymerase chain reaction profiling and small RNA sequencing. Plant Physiol. 150;1541–1555.
Rouphael, Y., M. Cardarelli, G. Colla, and E. Rea. 2008. Yield, mineral composition, water relations, and water use efficiency of grafted mini-watermelon plants under deficit irrigation. HortScience 43;730–736.
Rubio, V., F. Linhares, R. Solano, A.C. Martín, J. Iglesias, A. Leyva, and J. Paz-Ares. 2001. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev. 15;2122–2133.
Ruiz, J.M. and L. Romero. 1999. Nitrogen efficiency and metabolism in grafted melon plants. Sci. Hortic. 81;113–123.
Ruiz, J.M., A. Belakbir, I. López-Cantarero, and L. Romero. 1997. Leaf-macronutrient content and yield in grafted melon plants. A model to evaluate the influence of rootstock genotype. Sci. Hortic. 71;227–234.
Salehi, R., A. Kashi, J.-M. Lee, M. Babalar, M. Delshad, S.-G. Lee, and Y.-C. Huh. 2010. Leaf gas exchanges and mineral ion composition in xylem sap of iranian melon affected by rootstocks and training methods. HortScience 45;766–770.
Schachtman, D.P. and R. Shin. 2007. Nutrient sensing and signaling: NPKS. Annu. Rev. Plant Biol. 58;47–69.
Schwarz, D., Y. Rouphael, G. Colla, and J.H. Venema. 2010. Grafting as a tool to improve tolerance of vegetables to abiotic stresses: Thermal stress, water stress and organic pollutants. Sci. Hortic. 127;162–171.
Shin, R. 2011. Transcriptional regulatory components responding to macronutrient limitation. J. Plant Biol. 54;286–293.
Shin, R., R.H. Berg, and D.P. Schachtman. 2005. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol. 46;1350–1357.
Todd, C.D., P. Zeng, A.M.R. Huete, M.E. Hoyos, and J.C. Polacco. 2004. Transcripts of MYB-like genes respond to phosphorous and nitrogen deprivation in Arabidopsis. Planta 219;1003–1009.
Tsay, Y.-F., C.-C. Chiu, C.-B. Tsai, C.-H. Ho, and P.-K. Hsu. 2007. Nitrate transporters and peptide transporters. FEBS Letters 581 2290–2300.
Vance, C.P., C. Uhde Stone, and D.L. Allan. 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist 157 423–447.
Vidal, E.A. and R.A. Gutiérrez. 2008. A systems view of nitrogen nutrient and metabolite responses in Arabidopsis. Curr. Opin. Plant Biol. 11;521–529.
Vidal, E.A., V. Araus, C. Lu, G. Parry, P.J. Green, G.M. Coruzzi, and R.A. Gutiérrez. 2010. Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 107;4477–4482.
Voinnet, O. 2009. Origin, biogenesis, and activity of plant microRNAs. Cell 136;669–687.
Wang, J., M.Y. Park, L. Wang, Y.J. Koo, X. Chen, D. Weigel, and R.S. Poethig. 2011. miRNA control of vegetative phase change in trees. PLoS Genetics 7;e1002012.
Wu, H.J., Y.K. Ma, T. Chen, M. Wang, and X.J. Wang. 2012. PsRobot: a web-based plant small RNA meta-analysis toolbox. Nucleic Acids Res. 40:W22–W28.
Xu, Z., S. Zhong, X. Li, W. Li, S.J. Rothstein, S. Zhang, Y. Bi, and C. Xie. 2011. Genome-wide identification of microRNAs in response to low nitrate availability in maize leaves and roots. PLoS ONE 6;e28009.
Zeng, H., G. Wang, X. Hu, H. Wang, L. Du, and Y. Zhu. 2014. Role of microRNAs in plant responses to nutrient stress. Plant Soil 374;1005–1021.
Zeng, H.Q., Y.Y. Zhu, S.Q. Huang, and Z.M. Yang. 2010. Analysis of phosphorus-deficient responsive miRNAs and cis elements from soybean (Glycine max L.). J. Plant Physiol. 167;1289–1297.
Zhang, J., Y. Xu, Q. Huan, and K. Chong. 2009. Deep sequencing of Brachypodium small RNAs at the global genome level identifies microRNAs involved in cold stress response. BMC Genomics 10:449.
Zhao, M., H. Ding, J. Zhu, F. Zhang, and W. Li. 2011. Involvement of miR169 in the nitrogen-starvation responses in Arabidopsis. New Phytologist 190;906–915.
Zhao, M., H. Tai, S. Sun, F. Zhang, Y. Xu, and W. Li. 2012. Cloning and characterization of maize miRNAs involved in responses to nitrogen deficiency. PLoS ONE 7;e29669.
Zhou, M., D. Li, Z. Li, Q. Hu, C. Yang, L. Zhu, and H. Luo. 2013. Constitutive expression of a miR319 gene alters plant development and enhances salt and drought tolerance in transgenic creeping bentgrass. Plant Physiol. 161;1375–1391.
Zhu, Q., N.M. Upadhyaya, F. Gubler, and C.A. Helliwell. 2009. Over-expression of miR172 causes loss of spikelet determinacy and floral organ abnormalities in rice (Oryza sativa). BMC Plant Biol. 9:149.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors are contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, C., Yu, X., Bai, L. et al. Responses of miRNAs and their target genes to nitrogen- or phosphorus-deficiency in grafted cucumber seedlings. Hortic. Environ. Biotechnol. 57, 97–112 (2016). https://doi.org/10.1007/s13580-016-0092-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-016-0092-y