Abstract
Introduction
Despite the fact that high-dose radiotherapy is a main therapeutic modality in cancer treatment, recent evidence suggests that it might confer radioresistance. Hyper-radiosensitivity (HRS) is one of the important biological effects of low-dose ionizing radiation (LDIR) in mammalian cell lines. LDIR is considered as a promising assistant method of clinical cancer therapy. The purpose of this study was to evaluate the efficiency of intermittent LDIR followed by a high-dose radiation therapeutic approach compared with the conventional high-dose radiotherapy in the breast cancer MDA-MB-231 cell line.
Materials and methods
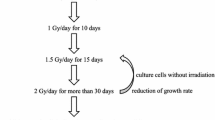
MDA-MB-231 cells were divided into four experimental groups—intermittent LDIR group: cells were irradiated for 10 fractions with a dose of 30 mGy at each time (interval 24 h) followed by 2 Gy, single LDIR group: cells have accepted a dose of 300 mGy LDIR and after 24 h a high dose of 2 Gy, high-dose ionizing radiation (HDIR) group: cells were exposed to a single high dose of 2 Gy, and control group.
Results
MTT and flow cytometry assay were used for cell proliferation and apoptosis after 24 h of the last irradiation dose (2 Gy). Also, we examined p21 and cespase3 gene expression by RT-qPCR. We observed that intermittent LDIR significantly increased the killing effect of radiotherapy (viability, 71.95 + 1.25%) (P < 0.01). The apoptosis is proposed to increase up to 32.55 + 0.07% in the intermittent LDIR that was markedly higher than those of other groups (P < 0.01). Caspase3 gene expression in this group was the highest (5.2-fold), 4.26-fold and 1.42-fold in single LDIR and HDIR, respectively. It was observed that the intermittent LDIR potentially decreases p21 expression in comparison with the challenge dose of 2 Gy (0.681-fold).
Conclusion
LDIR may result in HRS through a concurrent increase of apoptosis and a significant decrease in cell viability. The therapeutic effects of this approach should be further investigated in animal models.




Similar content being viewed by others
Abbreviations
- HDIR:
-
High-dose ionizing radiation
- HRS:
-
Hyper-radiosensitivity
- LDIR:
-
Low-dose ionizing radiation
- Int. L + H:
-
Intermittent LDIR followed by HDIR
- Single L + H:
-
Single LDIR followed by HDIR
References
Druesne-Pecollo N, Touvier M, Barrandon E, Chan DS, Norat T, Zelek L, Hercberg S, Latino-Martel P (2012) Excess body weight and second primary cancer risk after breast cancer: a systematic review and meta-analysis of prospective studies. Breast Cancer Res Treat 135(3):647–654
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Ferlay J, Héry C, Autier P, Sankaranarayanan R (2010) Global burden of breast cancer. In: Breast cancer epidemiology. Springer, pp 1–19
van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, van der Schueren E, Helle PA, van Zijl K, Bartelink H (2000) Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 92(14):1143–1150
Overgaard M, Jensen M-B, Overgaard J, Hansen PS, Rose C, Andersson M, Kamby C, Kjaer M, Gadeberg CC, Rasmussen BB, Blichert-Toft M, Mouridsen HT (1999) Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 353(9165):1641–1648. https://doi.org/10.1016/S0140-6736(98)09201-0
Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, Kjaer M, Gadeberg CC, Mouridsen HT, Jensen M-B, Zedeler K (1997) Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med 337(14):949–955. https://doi.org/10.1056/nejm199710023371401
Buchholz TA, Somerfield MR, Griggs JJ, El-Eid S, Hammond ME, Lyman GH, Mason G, Newman LA (2014) Margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: American Society of Clinical Oncology endorsement of the Society of Surgical Oncology/American Society for Radiation Oncology consensus guideline. J Clin Oncol Off J Am Soc Clin Oncol 32(14):1502–1506. https://doi.org/10.1200/jco.2014.55.1572
Morrow M, Van Zee KJ, Solin LJ, Houssami N, Chavez-MacGregor M, Harris JR, Horton J, Hwang S, Johnson PL, Marinovich ML, Schnitt SJ, Wapnir I, Moran MS (2016) Society of Surgical Oncology–American Society for Radiation Oncology–American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. Pract Radiat Oncol 6(5):287–295. https://doi.org/10.1016/j.prro.2016.06.011
Correa C, Harris EE, Leonardi MC, Smith BD, Taghian AG, Thompson AM, White J, Harris JR (2017) Accelerated partial breast irradiation: executive summary for the update of an ASTRO evidence-based consensus statement. Pract Radiat Oncol 7(2):73–79. https://doi.org/10.1016/j.prro.2016.09.007
Recht A, Comen EA, Fine RE, Fleming GF, Hardenbergh PH, Ho AY, Hudis CA, Hwang ES, Kirshner JJ, Morrow M, Salerno KE, Sledge GW, Solin LJ, Spears PA, Whelan TJ, Somerfield MR, Edge SB (2016) Postmastectomy radiotherapy: an American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology focused guideline update. Pract Radiat Oncol 6(6):e219–e234. https://doi.org/10.1016/j.prro.2016.08.009
Mayo CS, Urie MM, Fitzgerald TJ (2005) Hybrid IMRT plans—concurrently treating conventional and IMRT beams for improved breast irradiation and reduced planning time. Int J Radiat Oncol Biol Phys 61(3):922–932. https://doi.org/10.1016/j.ijrobp.2004.10.033
Gewirtz DA, Hilliker ML, Wilson EN (2009) Promotion of autophagy as a mechanism for radiation sensitization of breast tumor cells. Radiother Oncol 92(3):323–328
Goldstein M, Kastan MB (2015) The DNA damage response: implications for tumor responses to radiation and chemotherapy. Annu Rev Med 66:129–143
Karar J, Maity A (2009) Modulating the tumor microenvironment to increase radiation responsiveness. Cancer Biol Ther 8(21):1994–2001
Kitahara O, Katagiri T, Tsunoda T, Harima Y, Nakamura Y (2002) Classification of sensitivity or resistance of cervical cancers to ionizing radiation according to expression profiles of 62 genes selected by cDNA microarray analysis. Neoplasia 4(4):295–303
Hu T, Zhou R, Zhao Y, Wu G (2016) Integrin alpha6/Akt/Erk signaling is essential for human breast cancer resistance to radiotherapy. Sci Rep 6:33376. https://doi.org/10.1038/srep33376
Wang X, Liu R, Ma B, Yang KH, Tian J, Jiang L, Bai ZG, Hao XY, Wang J, Li J (2010) High dose rate versus low dose rate intracavity brachytherapy for locally advanced uterine cervix cancer. Cochrane Database Syst Rev (7)
Mettler FA, Sinclair WK, Anspaugh L, Selby PB, Edington C, Webster EW, Harley JH, Wyckoff HO, Ricks RC (1990) The 1986 and 1988 UNSCEAR reports: findings and implications. Health Phys 58(3):241–250
Liang X, So YH, Cui J, XU X, ZHAO Y, CAI L, LI W (2011) The low-dose ionizing radiation stimulates cell proliferation via activation of the MAPK/ERK pathway in rat cultured mesenchymal stem cells. J Radiat Res 52(3):380–386
Jiang H, Li W, Li X, Cai L, Wang G (2008) Low-dose radiation induces adaptive response in normal cells, but not in tumor cells: in vitro and in vivo studies. J Radiat Res 49(3):219–230
Yang G, Li W, Jiang H, Liang X, Zhao Y, Yu D, Zhou L, Wang G, Tian H, Han F (2016) Low-dose radiation may be a novel approach to enhance the effectiveness of cancer therapeutics. Int J Cancer 139(10):2157–2168
Liang X, Gu J, Yu D, Wang G, Zhou L, Zhang X, Zhao Y, Chen X, Zheng S, Liu Q (2016) Low-dose radiation induces cell proliferation in human embryonic lung fibroblasts but not in lung cancer cells: importance of ERK1/2 and AKT signaling pathways. Dose-Response 14(1):1559325815622174
Luckey TD (1982) Physiological benefits from low levels of ionizing radiation. Health Phys 43(6):771–789
Feinendegen L (2005) Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol 78(925):3–7
Olivieri G, Bodycote J, Wolff S (1984) Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science 223:594–598
Ballarini F, Biaggi M, Ottolenghi A, Sapora O (2002) Cellular communication and bystander effects: a critical review for modelling low-dose radiation action. Mutat Res Fundam Mol Mech Mutagen 501(1):1–12
Fernet M, Mégnin-Chanet F, Hall J, Favaudon V (2010) Control of the G2/M checkpoints after exposure to low doses of ionising radiation: implications for hyper-radiosensitivity. DNA repair 9(1):48–57
Marples B, Joiner M (1993) The response of Chinese hamster V79 cells to low radiation doses: evidence of enhanced sensitivity of the whole cell population. Radiat Res 133(1):41–51
Joiner M, Lambin P, Malaise E, Robson T, Arrand J, Skov K, Marples B (1996) Hypersensitivity to very-low single radiation doses: its relationship to the adaptive response and induced radioresistance. Mutat Res Fundam Mol Mech Mutagen 358(2):171–183
Wouters B, Skarsgard L (1994) The response of a human tumor cell line to low radiation doses: evidence of enhanced sensitivity. Radiat Res 138(1s):S76–S80
Wouters BG, Sy AM, Skarsgard LD (1996) Low-dose hypersensitivity and increased radioresistance in a panel of human tumor cell lines with different radiosensitivity. Radiat Res 146(4):399–413
Wouters BG, Skarsgard LD (1997) Low-dose radiation sensitivity and induced radioresistance to cell killing in HT-29 cells is distinct from the" adaptive response" and cannot be explained by a subpopulation of sensitive cells. Radiat Res 148(5):435–442
Enns L, Bogen KT, Wizniak J, Murtha AD, Weinfeld M (2004) Low-dose radiation hypersensitivity is associated with p53-dependent Apoptosis11U. S. Department of Energy, University of California Lawrence Livermore National Laboratory contract W-7405-Eng-48 (KT Bogen), National Cancer Institute (Canada) grant 013104 (M. Weinfeld), Alberta Cancer Board Bridge and Pilot grant R-418 (AD Murtha), and US Department of Energy Low-Dose Radiation Research Program (KT Bogen). Mol Cancer Res 2(10):557–566
Krueger SA, Joiner MC, Weinfeld M, Piasentin E, Marples B (2007) Role of apoptosis in low-dose hyper-radiosensitivity. Radiat Res 167(3):260–267. https://doi.org/10.1667/rr0776.1
Li SJ, Liang XY, Li HJ, Yang GZ, Li W, Li Z, Zhou L, Wen X, Yu DH, Cui JW (2018) Low-dose irradiation inhibits proliferation of the p53null type human prostate cancer cells through the ATM/p21 pathway. Int J Mol Med 41(1):548–554. https://doi.org/10.3892/ijmm.2017.3237
Joiner MC, Marples B, Lambin P, Short SC, Turesson I (2001) Low-dose hypersensitivity: current status and possible mechanisms. Int J Radiat Oncol Biol Phys 49(2):379–389
Yang X, Zhong D-N, Qin H, Wu P-R, Wei K-L, Chen G, He R-Q, Zhong J-C (2017) Caspase-3 over-expression is associated with poor overall survival and clinicopathological parameters in breast cancer: a meta-analysis of 3091 cases. Oncotarget 9(9):8629–8641. https://doi.org/10.18632/oncotarget.23667
Radwan RR, Abdel Fattah SM (2017) Mechanisms involved in the possible nephroprotective effect of rutin and low dose gamma irradiation against cisplatin-induced nephropathy in rats. J Photochem Photobiol B 169:56–62. https://doi.org/10.1016/j.jphotobiol.2017.02.022
Arenas M, Sabater S, Jimenez PL, Rovirosa A, Biete A, Linares V, Belles M, Panes J (2016) Radiotherapy for Graves’ disease. The possible role of low-dose radiotherapy. Rep Pract Oncol Radiother 21(3):213–218. https://doi.org/10.1016/j.rpor.2016.02.001
Gonc U, Cetinkaya M, Atabek M (2016) The effects of low-dose radiotherapy on fresh osteochondral allografts: an experimental study in rabbits. Acta Orthop Traumatol Turc 50(5):572–577. https://doi.org/10.1016/j.aott.2016.08.004
Chen HP, Tung FI, Chen MH, Liu TY (2016) A magnetic vehicle realized tumor cell-targeted radiotherapy using low-dose radiation. J Control Release 226:182–192. https://doi.org/10.1016/j.jconrel.2016.02.025
Yan S, Li X, Jin Q, Yuan J (2016) MicroRNA-145 sensitizes cervical cancer cells to low-dose irradiation by downregulating OCT4 expression. Exp Ther Med 12(5):3130–3136
Liu H, Zang C, Fenner M, Possinger K, Elstner E (2003) PPARγ ligands and ATRA inhibit the invasion of human breast cancer cells in vitro. Breast Cancer Res Treat 79(1):63–74
Chavez KJ, Garimella SV, Lipkowitz S (2010) Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast disease 32(1–2):35–48
Zhu X, Castellani RJ, Takeda A, Nunomura A, Atwood CS, Perry G, Smith MA (2001) Differential activation of neuronal ERK, JNK/SAPK and p38 in Alzheimer disease: the ‘two hit’ hypothesis. Mech Ageing Dev 123(1):39–46
Ghazali N, Shaw RJ, Rogers SN, Risk JM (2012) Genomic determinants of normal tissue toxicity after radiotherapy for head and neck malignancy: a systematic review. Oral Oncol 48(11):1090–1100
Suzuki K, Kodama S, Watanabe M (2001) Extremely low-dose ionizing radiation causes activation of mitogen-activated protein kinase pathway and enhances proliferation of normal human diploid cells. Cancer Res 61(14):5396–5401
Himoto T, Nomura T, Tani J, Miyoshi H, Morishita A, Yoneyama H, Haba R, Masugata H, Masaki T (2015) Exacerbation of insulin resistance and hepatic steatosis deriving from zinc deficiency in patients with HCV-related chronic liver disease. Biol Trace Elem Res 163(1–2):81–88
Wang Y, Li Y, Yang L, Yin D (2017) Intermittent low dose irradiation enhances the effectiveness of radio-and chemo-therapy for human colorectal adenocarcinoma cell line HT-29. Oncol Rep 38(1):591–597
Schwarz SB, Schaffer PM, Kulka U, Ertl-Wagner B, Hell R, Schaffer M (2008) The effect of radio-adaptive doses on HT29 and GM637 cells. Radiat Oncol 3(1):12
Jiang H, Xu Y, Li W, Ma K, Cai L, Wang G (2008) Low-dose radiation does not induce proliferation in tumor cells in vitro and in vivo. Radiat Res 170(4):477–487
Marples B, Collis SJ (2008) Low-dose hyper-radiosensitivity: past, present, and future. Int J Radiat Oncol Biol Phys 70(5):1310–1318
Li S-J, Liang X-Y, Li H-J, Li W, Zhou L, Chen H-Q, Ye S-G, Yu D-H, Cui J-W (2017) Low-dose irradiation promotes proliferation of the human breast cancer MDA-MB-231 cells through accumulation of mutant P53. Int J Oncol 50(1):290–296
Yan F, He Q, Hu X, Li W, Wei K, Li L, Zhong Y, Ding X, Xiang S, Zhang J (2013) Direct regulation of caspase3 by the transcription factor AP2alpha is involved in aspirin induced apoptosis in MDAMB453 breast cancer cells. Mol Med Rep 7(3):909–914. https://doi.org/10.3892/mmr.2013.1257
Soung YH, Lee JW, Kim SY, Park WS, Nam SW, Lee JY, Yoo NJ, Lee SH (2004) Somatic mutations of CASP3 gene in human cancers. Hum Genet 115(2):112–115
Chen K, Zhao H, Hu Z, Wang L-E, Zhang W, Sturgis EM, Wei Q (2008) CASP3 polymorphisms and risk of squamous cell carcinoma of the head and neck. Clin Cancer Res 14(19):6343–6349
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (Grant No.: U–96124).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Danyaei, A., Khanbabaei, H., Teimoori, A. et al. Effect of intermittent low-dose irradiation on the radiotherapy efficiency for MDA-MB-231 human breast adenocarcinoma cell line. J Radiat Oncol 8, 199–208 (2019). https://doi.org/10.1007/s13566-019-00388-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13566-019-00388-w