Abstract
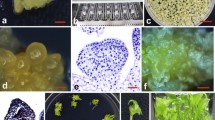
An efficient transformation protocol was developed for vanilla (Vanilla planifolia) using protocorm-like bodies (PLBs) derived from shoot tips as explants. Of the ten media tested, Murashige and Skoog (MS) medium containing 0.45 μM thidiazuron (TDZ) produced maximum PLBs per shoot tip. Genetic fidelity of PLB-derived plantlets was confirmed by random amplified polymorphic DNA (RAPD) using 23 random primers. PLBs were co-cultured with Agrobacterium tumefaciens strain EHA105 harbouring the binary vector pBI121 containing the β-glucuronidase (gusA) and neomycin phosphotransferase II (npt II) genes for 3 days in MS medium supplemented with acetosyringone and transferred to selective regeneration medium containing 4.43 μM benzyladenine (BA), 2.68 μM naphthalene acetic acid (NAA) supplemented with 50 mg l−1kanamycin and 250 mg l−1 cefotaxime. After 15 days of culture, the surviving explants were transferred to the same regeneration medium but with a higher concentration of kanamycin (75 mg l−1). Finally, explants surviving after 30 days were subjected to more stringent selection in the regeneration medium supplemented with 100 mg l−1 kanamycin. Strong β glucuronidase activity was detected in the transformed plantlets by histochemical assay. Integration of T-DNA into the nuclear genome of transgenic plants was confirmed by polymerase chain reaction and Southern hybridization, while expression of transgene was confirmed by northern hybridization. This protocol allows effective and high frequency transformation of vanilla.






Similar content being viewed by others
Abbreviations
- BA:
-
6-Benzyladenine
- GUS:
-
β-Glucuronidase
- NAA:
-
α-Naphthalene acetic acid
- MS:
-
Murashige and Skoog
- npt II:
-
Neomycin phosphotransferase II
- PLB:
-
Protocorm-like body
- PCR:
-
Polymerase chain reaction
- RAPD:
-
Random amplified polymorphic DNA
- TDZ:
-
Thidiazuron
- X-glcA:
-
5-bromo-4-chloro-3-indolyl-β-d-glucuronide
- YEP:
-
Yeast extract peptone medium
References
Barik DP, Mohapatra U, Chand PK (2005) Transgenic grasspea (Lathyrus sativus L.): factors influencing Agrobacterium-mediated transformation and regeneration. Plant Cell Rep 24:523–531
Besse P, Da Silva D, Bory S (2004) RAPD genetic diversity in cultivated vanilla: Vanilla planifolia, and relationships with V. tahitensis and V. pompona. Plant Sci 167:379–385
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidium thiocyanate-phenolchloroform extraction. Anal Biochem 162:156–159
Collen AMC, Jarl CI (1999) Comparison of different methods for plant regeneration and transformation of the legume Galega orientalis Lam. (goat’s rue). Plant Cell Rep 19:13–19
Cui J, Liu J, Deng M, Chen J, Henny RJ (2008) Plant regeneration through protocorm-like bodies induced from node explants of Syngonium podophyllum White Butterfly. Hortic Sci 43:2129–2133
Davidonis G, Knorr D (1991) Callus formation and shoot regeneration in Vanilla planifolia. Food Biotechnol 5:59–66
Dequaire J (1976) L'amélioration du vanillier à Madagascar J AgricTrop Bot Appl 23:140–158
Divakaran M, Nirmalbabu K, Ravindran PN, Peter KV (2006) Interspecific hybridization in vanilla and molecular characterization of hybrids and selfed progenies using RAPD and AFLP markers. Sci Hortic 108:414–422
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:12–14
Dutta I, Saha P, Das S (2008) Efficient Agrobacterium-mediated genetic transformation of oilseed mustard Brassica juncea (L.) Czern. using leaf piece explants. In Vitro Cell Dev Biol Plant 44:401–411
Hei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of boundaries of the T-DNA. Plant J 6:271–282
Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2:208–218
Husnain T, Malik T, Riazuddian S, Gordon MP (1997) Studies on expression of marker genes in chickpea. Plant Cell Tissue Organ Cult 49:7–16
Ishida Y, Saito H, Ohta S, Hiei Y, Komari T, Kumashiro T (1996) High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nat Biotechnol 14:745–750
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion sytem. Plant Mol Biol Rep 5:387–405
Kononowicz H, Janick J (1984) In vitro propagation of Vanilla planifolia. Hortic Sci 19:58–59
Kotsuka K, Tada Y (2008) Genetic transformation of golden pothos (Epipremnum aureum) mediated by Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult 95:305–311
Malabadi RB, Nataraja K (2007) Genetic transformation of Vanilla planifolia by Agrobacterium tumefaciens using shoot tip sections. Res J Bot 2:86–94
Men S, Ming X, Wang Y, Liu R, Wei C, Li Y (2003) Genetic transformation of two species of orchid by biolistic bombardment. Plant Cell Rep 21:592–598
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Muthukumar B, Mariamma M, Veluthambi K, Gnanam A (1996) Genetic transformation of cotyledon explants of cowpea (Vigna unguiculata L. Walp) using Agrobacterium tumefaciens. Plant Cell Rep 15:980–985
Nandakumar R, Chen L, Rogers SMD (2007) A stable and reproducible transformation system for the wetland monocot Juncus accuminatus (bulrush) mediated by Agrobacterium tumefaciens. In Vitro Cell Dev Biol Plant 43:187–194
Perez-Clemente R, Perez-Sanjuan A, Garzia-Ferriz L, Beltran J, Canas L (2004) Transgenic peach plants (Prunus persica L.) produced by genetic transformation of embryo sections using the green fluorescent protein (GFP) as in vivo marker. Mol Breed 14:419–427
Philip VJ, Nainar SAZ (1986) Clonal propagation of Vanilla planifolia (Salisb) Ames using tissue culture. J Plant Physiol 122:211–215
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Shekhawat UKS, Ganapathi TR, Srinivas L, Bapat VA, Rathore TS (2008) Agrobacterium-mediated genetic transformation of embryogenic cell suspension cultures of Santalum album L. Plant Cell Tissue Organ Cult 92:261–271
Silva J, Singh N, Tanaka M (2006) Priming biotic factors for optimal protocorm-like body and callus induction in hybrid Cymbidium (Orchidaceae), and assessment of cytogenetic stability in regenerated plantlets. Plant Cell Tissue Organ Cult 84:135–144
Singh ND, Sahoo L, Sonia JPK (2002) In vitro shoot organogenesis and plant regeneration from cotyledonary node and leaf explants of pigeon pea (Cajanus cajan L. Millsp). Physiol Mol Biol Plant 8:113–140
Soto Arenas MA (2003) Vanilla. In: Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN (eds) Genera orchidacearum: Orchidoideae. Oxford University Press, USA
Acknowledgment
The authors are grateful to the Director and Head, Division of Crop Protection, IISR, Calicut for providing facilities. This piece of work forms part of the PhD research work of R.S.T.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Retheesh, S.T., Bhat, A.I. Genetic transformation and regeneration of transgenic plants from protocorm-like bodies of vanilla (Vanilla planifolia Andrews) using Agrobacterium tumefaciens . J. Plant Biochem. Biotechnol. 20, 262–269 (2011). https://doi.org/10.1007/s13562-011-0057-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-011-0057-2